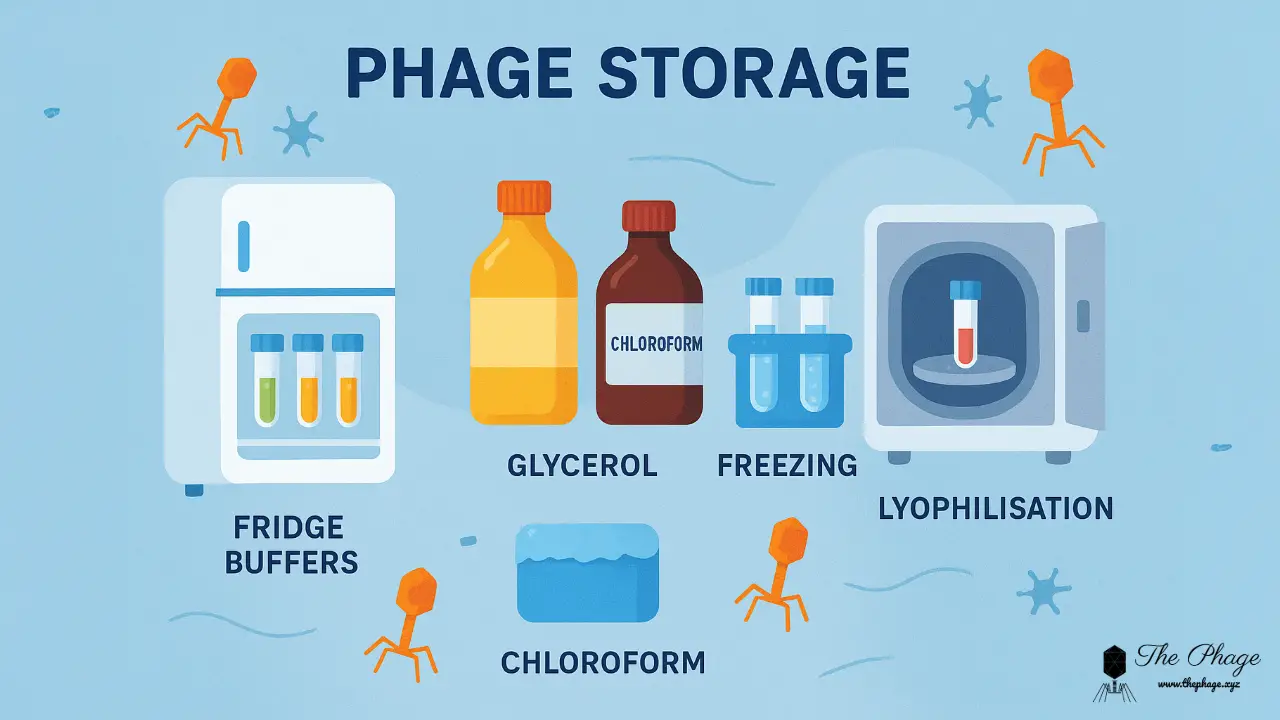
SM buffer is primarily used in biology laboratories and, like any other buffer, its primary function is to stabilize the pH. It can withstand minor pH changes, thereby increasing the stability of biological materials or molecules. When carrying out an experiment, it is critical to keep all other variables constant and only vary the variables that are being tested. This emphasizes the importance of maintaining pH stability through dependable and consistent methods such as buffer systems. One of them is SM buffer, which is used for routine phage suspension manipulation. In handling bacteriophages, buffers with and without gelatin can be used.
| Reagent | Amount | Final concentration |
|---|---|---|
| NaCl | 5.8 g | 100 mM |
| MgSO4•7H2O | 2 g | 8 mM |
| Tris-Cl (1 M, pH 7.5) | 50 ml | 50 mM |
| H2O | to 1 liter | |
To prepare 1 liter of SM buffer, dissolve the NaCl and MgSO4•7H2O in 800 ml of H2O; add the Tris-Cl, and adjust the volume to 1 liter with H2O. Sterilize the buffer by autoclaving for 20 minutes at 15 psi (1.05 kg/cm2) on the liquid cycle. After the solution has cooled, dispense 50-ml aliquots into sterile containers. SM buffer may be stored indefinitely at room temperature. Discard each aliquot after use to minimize the chance of contamination. SM buffer may also be prepared with 0.002% (w/v) gelatin; see the recipe for SM buffer with gelatin. To prepare SM Buffer with gelatin click here and to prepare Tris-cl click here


[…] prepare SM Buffer without gelatin click here and to prepare Tris-cl click […]