
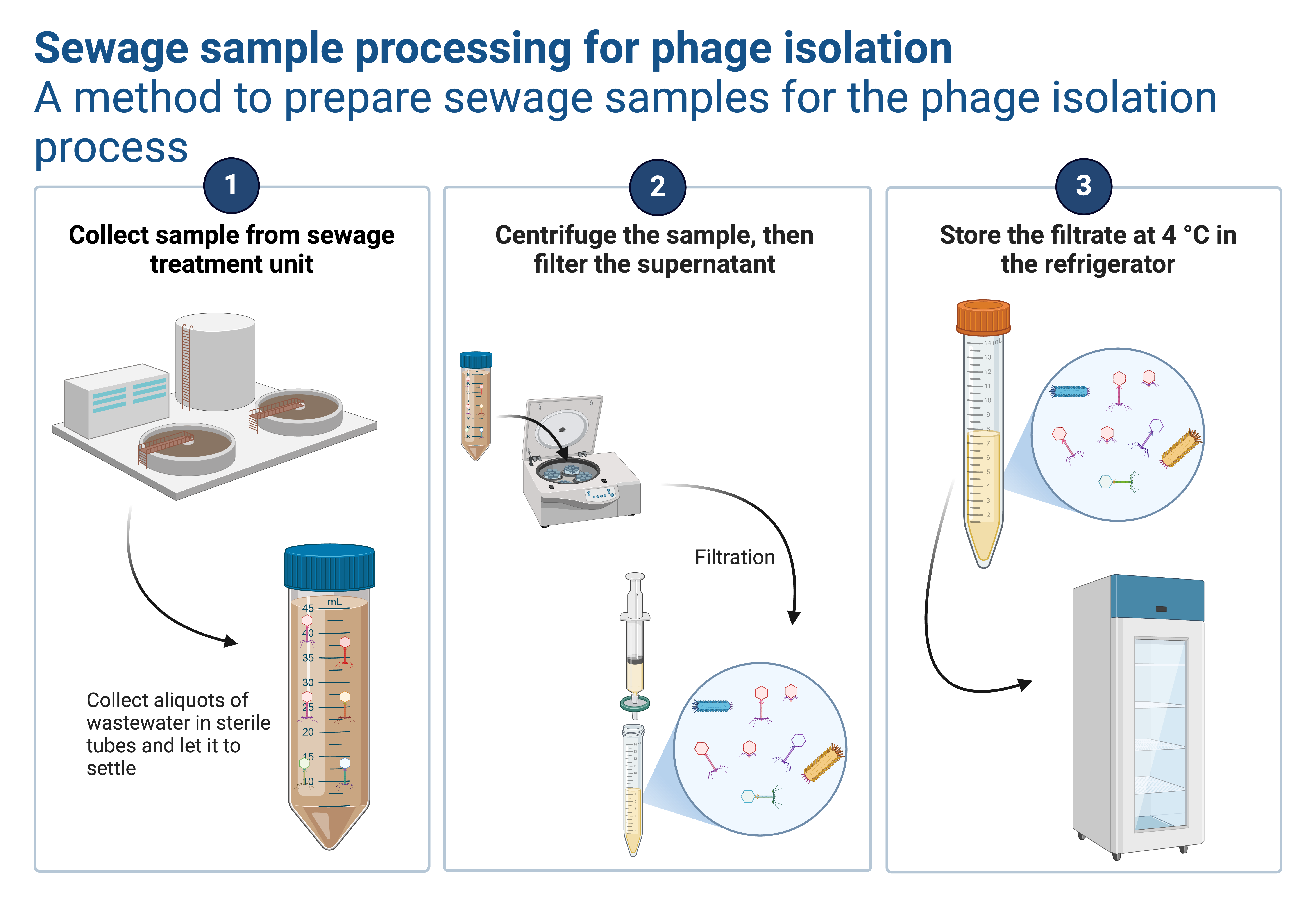
Preparation of an overlay
This method creates a homogeneous lawn of bacteria within a thin layer of agar across the surface of a plate. Bacteria are added to a soft top agar (0.65% agar, rather than the standard 1.5 % for agar plates) that has been melted at 100°C and cooled to 45°C. This temperature is warm enough to keep the agar liquid but cool enough not to kill the bacteria (for some time). The melted agar/bacterial suspension is mixed and poured evenly across the top of an agar plate before solidifying.
The bacteria spread across the top agar will grow to form a uniformly turbid lawn. If a freshly seeded lawn is exposed to various antibacterial agents and then incubated at 37°C, any inhibition of bacterial growth will result in a decrease in the turbidity of the property near the agent: the greater the antibacterial action, the wider the zone of inhibition. Thus, the width of the zone of inhibition around the agent can be used to determine its antibacterial strength.
EQUIPMENT:
- sterile capped 13×100 mm tubes
- sterile pipettes (0.1, 1.0 mL) or
- sterile tips for Displacement pipetter
- hot block or water bath, 45°C, warmed up
- vortex mixer
- Bunsen Burner/spirit burner
SUPPLIES:
- Melted top agar, about 60°C
- Fresh overnight culture of host bacteria
- Pre-warmed nutrient agar plates (or tryptone soy agar plates, Luria Bertani (good for phage work), or any general media)
- Dishpan or bucket with diluted Lysol into which to discard used tubes (You can use other decontaminant/disinfectant as per your laboratory policy)
PROTOCOL:
- Inoculate about 3-4 mL of nutrient broth or tryptone soy broth with the desired host bacterium and incubate for 18- 24 hours.
- Pipet about 4 mL of hot melted top agar into sterile capped 13×100 mm tubes in a 45°C hot block or water bath (At this temperature the overlay is not supposed to solidify).
- Pipet about 0.1 mL of an overnight bacterial culture of the host into the melted agar.
- Overlay technique: a. Vortex to mix the bacteria into the melted top agar using a vortexer b. Immediately pour out onto a pre-warmed agar plate to empty the tube. c. To evenly distribute, immediately tilt back and forth and gently shake. Avoid bubbles and stop agitating before the agar gels. Allow the setting undisturbed to fully solidify.
- When fully gelled, you can now use your plate for any function as your requirements. For bacteriophages, this is the stage where the spotting procedure happens.
- Invert, and incubate overnight at 37°C (This depends on bacteria of interest).
- The following morning, read the plates. The presence and activity of bacteriophages will be directly reflected in the clearance of the bacterial lawn.
For more protocols please visit The Phage protocols
The Double–Layer Agar (DLA) technique is extensively used in phage research to enumerate and identify phages and isolate mutants and new phages.

Leave a Reply
You must be logged in to post a comment.