SNIPR Biome ApS has made a groundbreaking discovery in the field of microbiology, paving the way for a new era of microbial gene therapy. Nature Biotechnology has recently published the results of SNIPR’s preclinical work on SNIPR001, a revolutionary CRISPR-armed phage therapeutic designed to specifically target and remove E. coli, including antibiotic-resistant strains, in the human gastrointestinal tract.
The increasing prevalence of antibiotic-resistant pathogens poses a major global challenge to the medical community. Current medical practices rely on increasingly aggressive therapies, which can sometimes result in patients experiencing life-threatening infections caused by antibiotic-resistant bacteria. This issue is often underestimated in contemporary healthcare systems, and the threat of antimicrobial resistance could lead to significant healthcare challenges in the future.
SNIPR001 is a potential game-changer, designed to prevent infections from spreading into the bloodstream by specifically targeting and eradicating E. coli in the gut. This breakthrough therapy has the potential to revolutionize the way we prevent and treat infections and could serve as a model for developing similar therapies that target other life-threatening pathogens. SNIPR Biome’s research has been validated through the publication of their findings in Nature Biotechnology, opening up exciting possibilities for the future of microbial gene therapy.
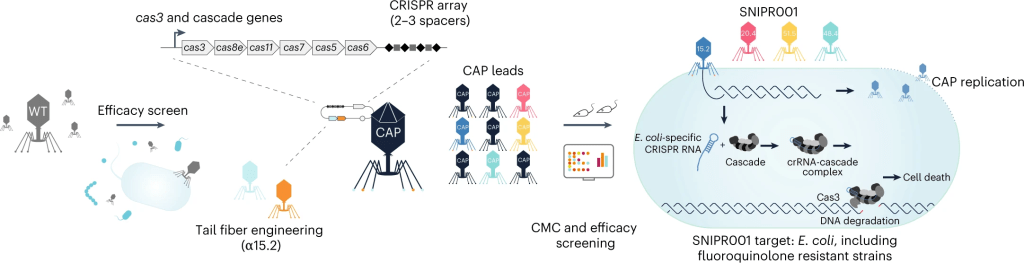
Antibiotic treatments have detrimental effects on the microbiome and lead to antibiotic resistance. To develop a phage therapy against a diverse range of clinically relevant Escherichia coli, SNIPR Biome screened a library of 162 wild-type (WT) phages and identified eight phages with broad coverage of E. coli, complementary binding to bacterial surface receptors, and the capability to stably carry inserted cargo. The selected phages were then engineered with tail fibers and CRISPR-Cas machinery to specifically target E. coli. SNIPR Biome’s research shows that engineered phages target bacteria in biofilms, reduce the emergence of phage-tolerant E. coli, and out-compete their ancestral WT phages in coculture experiments. A combination of the four most complementary bacteriophages, called SNIPR001, is well tolerated in both mouse models and minipigs and reduces E. coli load in the mouse gut better than its constituent components separately. SNIPR001 is currently undergoing a Phase 1 trial in the US to evaluate its safety and efficacy in reducing E. coli in the gut without disturbing the overall gut microbiome (NCT05277350).
Leave a Reply
You must be logged in to post a comment.