SNIPR Biome, a Danish microbiome technology company, has unveiled promising results from the initial phase of their groundbreaking CRISPR-based phage therapy targeting Escherichia coli in the gastrointestinal tract.
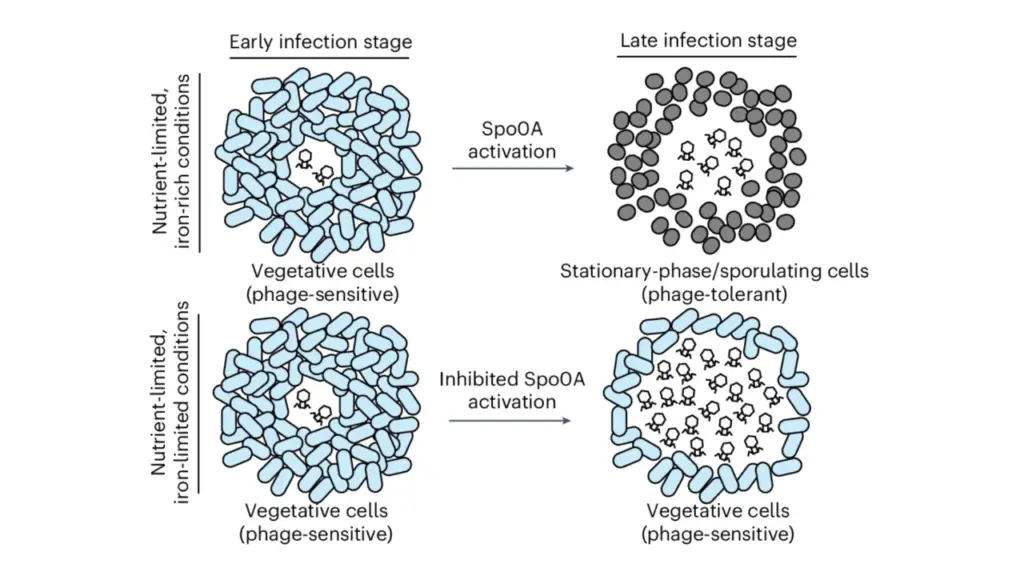
Imagine a team of microscopic superheroes armed with advanced DNA editing technology. That’s SNIPR001, a powerful blend of four bacteriophages (tiny viruses that specialize in obliterating bacteria) equipped with CRISPR/Cas technology. Its mission? To selectively eradicate E. coli in the gut, even the stubborn antibiotic-resistant strains. And guess what? SNIPR001 is already showing remarkable potential.
After acing its animal trials with flying colors, the remarkable product, SNIPR001, has emerged as a potential game-changer. The exciting breakthrough in animal trials laid the foundation for its momentous journey, leading to the eagerly awaited human trial. Now, with the spotlight firmly fixed on this innovative marvel, we delve into its fascinating story, tracing its path from animal triumphs to the groundbreaking human trial.
The eagerly anticipated phase 1 trial, conducted on 36 individuals, aimed to assess the safety and effectiveness of SNIPR001. The results were nothing short of impressive. Participants who received oral doses of SNIPR001 over a span of 7 days experienced only mild to moderate side effects, demonstrating excellent tolerability. But that’s not all. The treatment also exhibited a numerical reduction in gut E. coli levels, highlighting its ability to combat this problematic bacterium.
This groundbreaking therapy is set to provide a lifeline to patients grappling with hematologic cancers, such as lymphoma and leukemia. These individuals often undergo hematopoietic stem-cell transplants, leaving them vulnerable to bloodstream infections resulting from E. coli moving from the gut into the blood. Unfortunately, the most common antibiotic treatment, fluoroquinolones, is rendered ineffective against fluoroquinolone-resistant E. coli strains. Moreover, it tends to harm the gut microbiome, wiping out the beneficial bacteria crucial for overall health.
“With the combined killing effects of bacteriophages and CRISPR-Cas technology, SNIPR001 has demonstrated the ability to target and eliminate antibiotic-resistant E. coli strains in the gut, providing a safe alternative to traditional treatments that do not work against antibiotic-resistant strains, while sparing the rest of the gut microbiome.”
Christian Grondahl, co-founder, and CEO of SNIPR Biome
Buoyed by these encouraging results, SNIPR Biome is eagerly marching forward to conduct further clinical studies. Their ultimate goal? To improve patient outcomes by revolutionizing the field of antimicrobial resistance. The next phase of trials will focus on investigating whether SNIPR001 can effectively reduce the incidence of E. coli bloodstream infections in cancer patients.
In recognition of its groundbreaking potential, SNIPR Biome secured $3.9 million in funding from CARB-X (the Combating Antibiotic Resistant Bacteria Biopharmaceutical Accelerator) in May 2021. This financial support serves as a testament to the growing excitement surrounding SNIPR001 and its potential to reshape the landscape of microbial therapies.
As SNIPR Biome embarks on the next chapter of its groundbreaking journey, there is a glimmer of hope that this innovative therapy will provide a safe and effective solution against antibiotic-resistant strains of E. coli, all while preserving the delicate balance of the gut microbiome.


Comments