A team of researchers from the University of Geneva (UNIGE) and the Geneva University Hospitals (HUG) has achieved a significant breakthrough by using bacteriophages to successfully treat a patient with a chronic bacterial lung infection that was resistant to antibiotics. This groundbreaking achievement was made possible through a personalized and multidisciplinary approach, which involved identifying a bacteriophage that specifically targeted the patient’s multidrug-resistant bacteria. While phages are being explored as a potential solution to combat antibiotic resistance, there is still much work to be done in terms of selecting phages tailored to individual patients, establishing treatment protocols, understanding potential side effects, and preventing the emergence of phage-resistant strains. This exceptional case of personalized therapy provides valuable insights and has been published in the journal Nature Communications.
In this study, the team from HUG and UNIGE utilized phagotherapy to treat a 41-year-old patient suffering from a chronic lung disease caused by a multidrug-resistant strain of the bacterium Pseudomonas aeruginosa. The patient had been hospitalized at HUG for six months, relying on continuous intravenous antibiotic therapy with no signs of improvement. As a last-resort measure, the patient received an experimental phage treatment authorized on compassionate grounds. This treatment proved to be successful, allowing the patient to be discharged from the hospital, regain independence, and return to work.
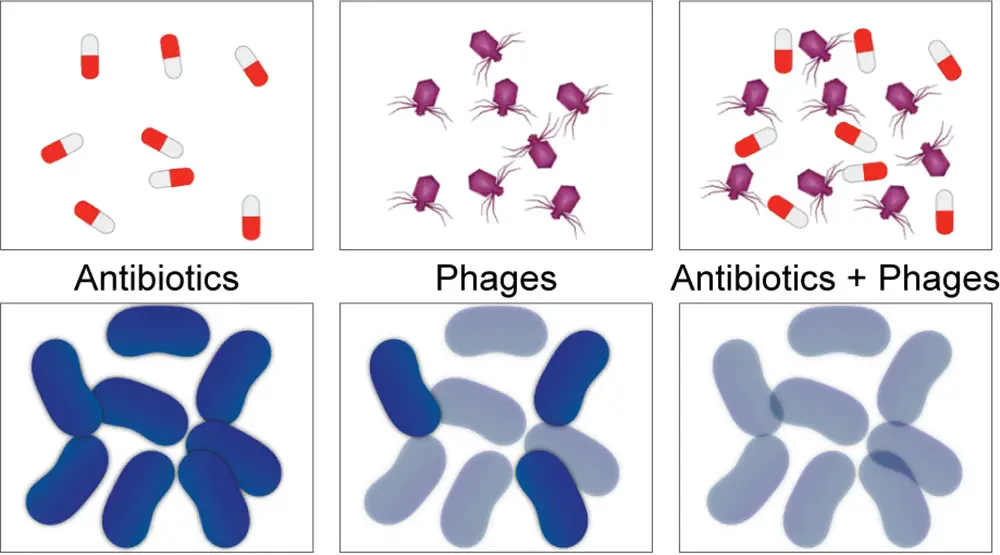
Phage therapy involves the use of bacteriophages, which are natural viruses that target specific bacterial strains without infecting human cells. When combined with antibiotics, phages can help overcome antibiotic resistance. While this approach shows promise, it requires further scientific and clinical research to develop effective, safe, and approved treatments, which is not yet the case.
The success of the therapy relied on a highly individualized approach. Dr. Thilo Köhler, a research group co-leader in the Department of Microbiology and Molecular Medicine at the UNIGE Faculty of Medicine, emphasized the importance of selecting the appropriate phage specific to the patient’s bacterial strain before initiating phage therapy. The team isolated the bacteria from the patient’s respiratory secretions and determined its type, genetic profile, and resistance patterns to both antibiotics and a phage bank. Despite an extensive search through various phage banks at HUG, the Lausanne University Hospital (CHUV), and the European Phage Centre at the Queen Astrid Military Hospital in Brussels, a suitable phage was ultimately identified at Yale University in the United States.
The phages were administered to the patient through aerosol delivery while intravenous antibiotics were continued. The patient’s recovery was remarkable, with no reported side effects, confirming the efficacy and safety of the phage treatment. Detailed monitoring of the patient’s secretions demonstrated that phage replication was limited to the targeted bacteria, without the emergence of more resistant or harmful bacteria. It is important to note that phage therapy is used in conjunction with antibiotic therapy, not as a replacement, as emphasized by Prof. Christian van Delden, Staff Physician in the Division of Infectious Diseases at HUG and Full Professor in the Department of Medicine at the UNIGE Faculty of Medicine. He underscored the significance of exploring new strategies to combat antibiotic resistance and highlighted the success of the translational approach employed in this study, from patient to laboratory and back to the patient. The Geneva team expressed gratitude for the collaboration with the American group, which generously provided the phages at no cost, without which this life-saving treatment would not have been possible.
Antibiotic resistance poses a global health emergency, resulting in millions of deaths worldwide each year, with an estimated 1.7 million deaths in 2019 according to the World Health Organization (WHO) Report. Bacteria possess inherent properties that contribute to this issue. In nature and within the human body, bacteria exist
as part of an ecosystem alongside other bacterial species, fungi, and bacteriophages. Bacteria have the ability to evolve rapidly through mutations, allowing them to adapt to their environment and evade antibiotics. Prolonged or repeated exposure to antibiotics leads to the selection of resistant bacterial strains. Phage therapy holds promise as one of the strategies to address this significant threat to human health.
Read the full scientific article here
Köhler, T., Luscher, A., Falconnet, L. et al. Personalized aerosolized bacteriophage treatment of chronic lung infection due to multidrug-resistant Pseudomonas aeruginosa. Nat Commun 14, 3629 (2023). https://doi.org/10.1038/s41467-023-39370-z