In a groundbreaking international endeavor, the Israeli Phage Therapy Center (IPTC), under the leadership of Prof. Ran Nir-Paz from the Hadassah Hebrew University Medical Center and Prof. Ronen Hazan from The Hebrew University of Jerusalem’s Faculty of Dental Medicine, has undertaken a pioneering study. The study focused on employing phage PASA16, a specialized viral agent, on a compassionate basis to combat formidable Pseudomonas aeruginosa (P. aeruginosa) infections. The results have been nothing short of promising, boasting an impressive 86.6% success rate.
This unparalleled research, involving 16 patients grappling with persistent infections, marks a pivotal moment in the field. The findings have unearthed the potential effectiveness of PASA16 phage therapy in confronting the challenging P. aeruginosa infections that have long evaded conventional treatments. This success story not only paves the way for forthcoming clinical trials but also serves as a beacon guiding further exploration into phage therapy’s role as a potent alternative against antibiotic-resistant infections. The study has been published in the prestigious journal Med, further cementing its significance.
The concept of phage therapy, which deploys specialized viruses to combat infections, has emerged as a groundbreaking addition to the arsenal of conventional antibiotics. Even the well know organizations such as American CDC have given the go-ahead in using phage therapy. While previous clinical trials in this arena have been somewhat restricted, recent cases of compassionate phage therapy have ignited hope, although solid evidence remains scarce.
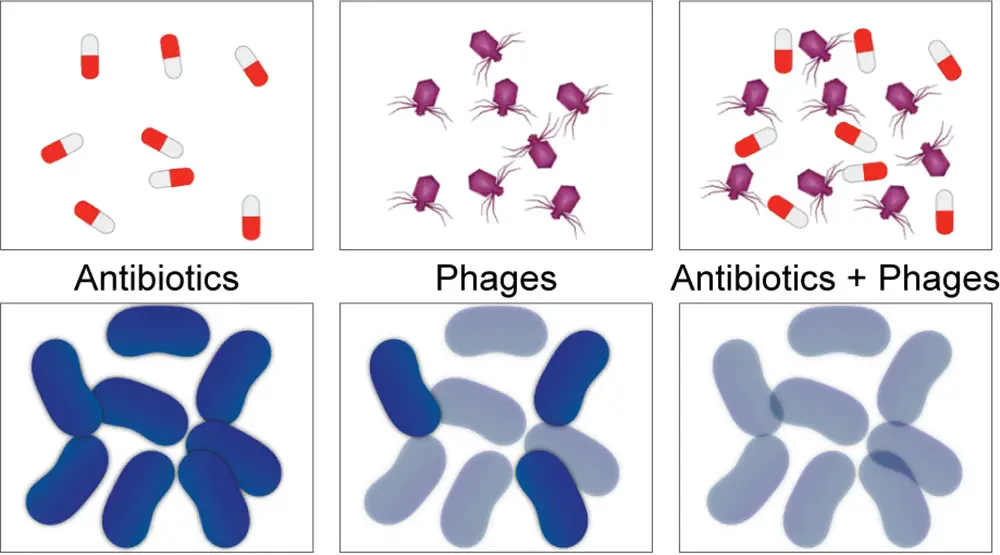
In a remarkable leap forward, this study illuminates the synergistic potential of phages working in tandem with antibiotics to tackle the notoriously stubborn P. aeruginosa infections that have defied conventional interventions.
Preceding the treatment, comprehensive testing of P. aeruginosa isolates from patients was undertaken, enabling personalized and sensitive application of the PASA16 phage. Throughout the PASA16 phage treatment, only minor and manageable side effects surfaced. Impressively, an overwhelming 86.6% success rate was recorded, with 13 out of 15 patients yielding favorable clinical outcomes based on available data.
This triumph underscores the viability of combining PASA16 phage therapy with antibiotics as a promising avenue for individuals who previously encountered treatment roadblocks. The treatment duration varied from eight days to six weeks, with the majority falling within the two-week range and involving one- to twice-daily regimens, thus providing an efficient and time-sensitive option.
To summarize, this compassionate case series stands as a resounding testament to the efficacy of PASA16 phage therapy in tackling the tenacious P. aeruginosa infections. By delineating potential clinical protocols, this study serves as the stepping stone for future trials. The triumph witnessed serves as a clarion call for extended research and deeper exploration of phage therapy as a formidable adjunct to counter antibiotic-resistant infections.
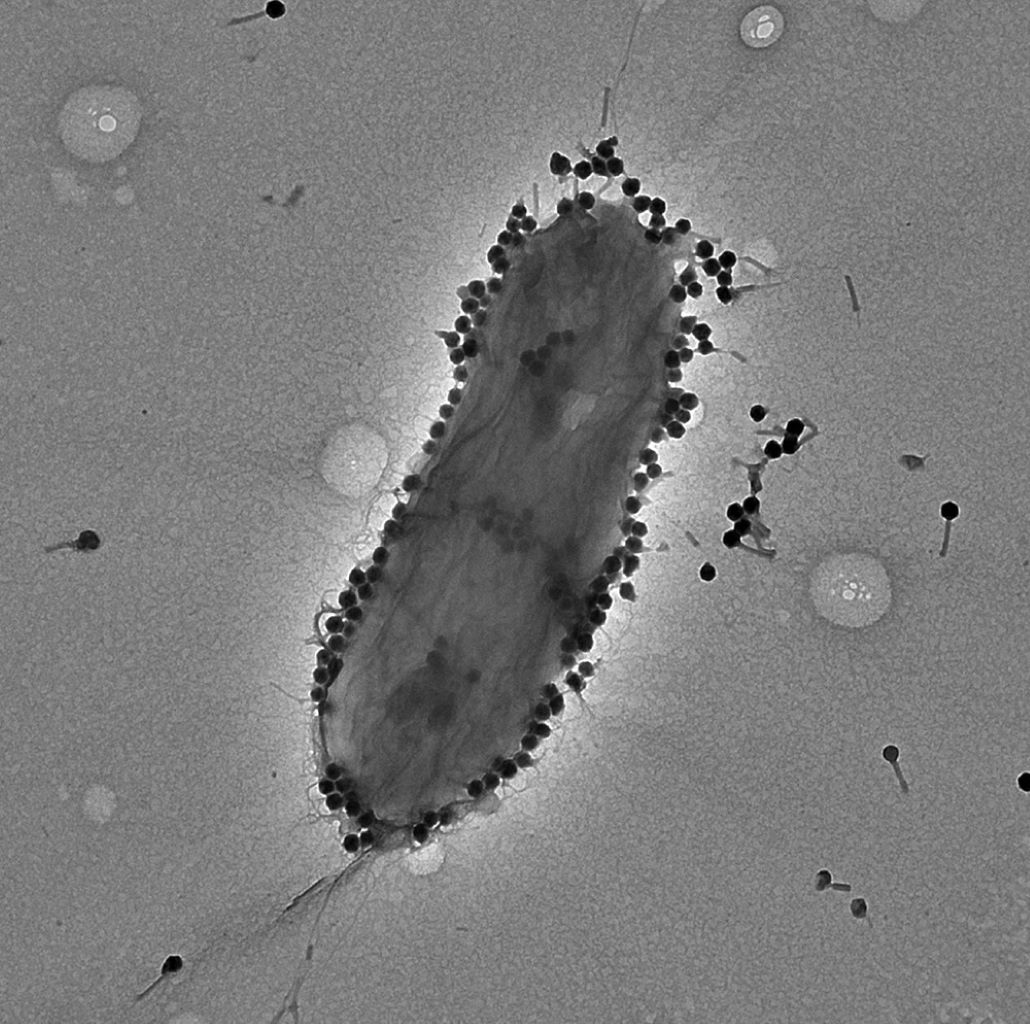
Pseudomonas aeruginosa, a versatile bacterium endemic in various environments such as soil, water, plants, and human microbiota, assumes the dual role of both pathogen and opportunistic agent. It strikes particularly at individuals with compromised immune systems or underlying health conditions. Known for causing intricate and potentially life-threatening infections, this bacterium poses a formidable challenge within healthcare settings.
Its impact can range from mild to severe, affecting diverse bodily regions such as the lungs, urinary tract, skin, and wounds. A frequent culprit in hospital-acquired infections, especially in immunocompromised patients or those reliant on mechanical ventilation or invasive devices, P. aeruginosa’s penchant for forming protective biofilms exacerbates the treatment process, at times necessitating a blend of antibiotics and innovative therapies like phage therapy. Stringent infection control measures within healthcare facilities stand as a crucial bulwark against its persistence.
The study artfully compiles a wealth of clinical data from 15 of the 16 patients treated with the phage PASA16, providing an incisive evaluation of its efficacy in combating infections. Donated by the American phage company “Adaptive Phage Therapeutics,” the PASA16 phage was administered through diverse methods, encompassing intravenous administration, localized application at infection sites, and topical usage. The dataset predominantly encompasses patients wrestling with osteoarticular and foreign-device-associated infections.
As the global battle against antibiotic resistance rages on, this compassionate use case series represents a momentous leap towards unearthing phage therapy’s potential as a lifeline for individuals plagued by resilient P. aeruginosa infections.
Phage therapy has experienced a renaissance as a plausible solution for infections resistant to antimicrobial interventions. Unlike broad-spectrum antibiotics, phages possess the unique ability to specifically target other microorganisms. While compassionate use cases for phage therapy have materialized, robust clinical trials remain somewhat elusive.
This study takes the lead by chronicling the most extensive case series to date, focusing on consecutive patients confronting severe P. aeruginosa infections. Under compassionate use circumstances, a single specific phage, PASA16, was employed. All 16 patients were treated following a thorough analysis confirming their infective agent’s susceptibility to both the phage on its own and when paired with antibiotics. The findings lay bare a favorable outcome in over 80% of treated patients, accompanied by minimal side effects. Armed with these results, the logical next steps involve the propagation of additional compassionate use cases and the formulation of comprehensive clinical trial protocols.
This study was published Ran Nir-Paz, Refractory Pseudomonas aeruginosa infections treated with Phage PASA16: a compassionate use case series, Med (2023). DOI: 10.1016/j.medj.2023.07.002. www.cell.com/med/fulltext/S2666-6340(23)00225-8


Comments