In a recent study published in the open-access journal PLOS Biology, a team of researchers led by Dr. Barr from Monash University in Victoria, Australia, have put forth an intriguing proposition. They suggest that mammalian cells actively internalize phages as a valuable resource, all in the name of enhancing their growth and ensuring their survival.
While the interactions between phages and bacteria have undergone extensive scrutiny, the influence of bacteriophages on the intricate web of mammalian cellular and immunological processes has largely remained uncharted territory.
To navigate this complex relationship between mammalian cells’ immune responses and their interactions with phages, scientists conducted experiments employing the well-studied T4 phage in vitro. They meticulously examined the cellular responses using luciferase reporter and antibody microarray assays, utilizing a phage-free supernatant as a basis for comparison.
The results, quite surprisingly, unveiled a revelation: T4 phages did not spark DNA-mediated inflammatory pathways within mammalian cells. Instead, they set in motion a sequence of signaling events that actively spurred cellular growth and fortified the cells’ resilience. It’s important to note that additional research is required to unveil the precise motives behind the cellular adoption of phage particles as a resource, and whether this behavior has evolved over time to bestow specific advantages.
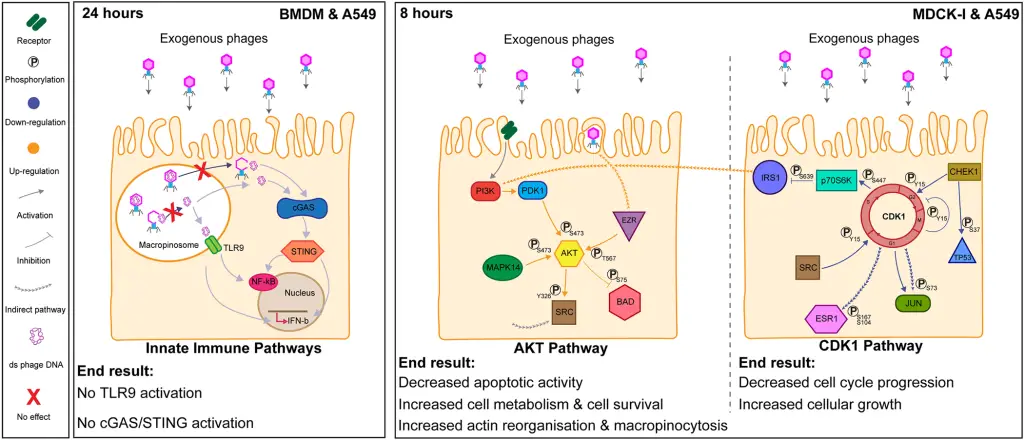
The study further disclosed that mammalian cells internalize bacteriophages, employing them as a valuable resource to amplify cellular growth and survival. The researchers conducted meticulous investigations, employing highly purified T4 phages devoid of bacterial contaminants. They observed swift internalization of T4 phages by mammalian cells, leading to their accumulation within macropinosomes. Notably, these phages refrained from activating the inflammatory DNA response pathways TLR9 or cGAS-STING.
This exploratory study casts a bright spotlight on the profound influence of phages on mammalian systems, with potential implications rippling across diverse fields, including human health, immunology, phage therapy, and microbiome research. As well as offering fresh perspectives on the potential benefits that bacteriophages might offer to their mammalian hosts. Given the escalating use of phage therapy in addressing antibiotic-resistant infections, this discovery holds particular significance.
What becomes evident through this pioneering research is the intricate, ever-evolving relationship between bacteriophages and their mammalian hosts, providing us with a glimpse into a realm of symbiosis that remains ripe for further exploration.
Following an 8-hour incubation with T4 phages, the researchers utilized antibody microarray assays to detect changes in the expression and phosphorylation levels of human signaling proteins. The results revealed that T4 phages triggered AKT-dependent pathways, resulting in heightened cell metabolism, enhanced cell survival, and a reorganization of the cellular actin structure—crucial for processes like macropinocytosis. These interactions also led to the down-regulation of CDK1 and its downstream effects, halting cell cycle progression and fostering cellular growth through an extended G1 phase.
This study casts a bright spotlight on the profound influence of phages on mammalian systems, with potential implications rippling across diverse fields, including human health, immunology, phage therapy, and microbiome research. Their groundbreaking research offers fresh perspectives on the potential benefits that bacteriophages might offer to their mammalian hosts. Given the escalating use of phage therapy in addressing antibiotic-resistant infections, this discovery holds particular significance.
What becomes evident through this pioneering work is the intricate, ever-evolving relationship between bacteriophages and their mammalian hosts, providing us with a glimpse into a realm of symbiosis that remains ripe for further exploration.
In essence, this study underscores that highly purified T4 phages abstain from provoking DNA-mediated inflammatory responses within mammalian cells. Instead, they set in motion protein phosphorylation cascades that fuel cellular growth and survival. These findings strongly imply that mammalian cells actively internalize bacteriophages as a valuable resource, enriching their own growth and metabolic processes.
For more in-depth information, you can explore the full scientific article here.

Leave a Reply
You must be logged in to post a comment.