Scientist from Stanford University, Stanford, CA, United States published a recent paper in a peer reviewed journal, Frontiers in Cellular and Infection Microbiology. In this groundbreaking study, scientists delve into the intricate relationship between our immune system and the bacterium Pseudomonas aeruginosa, a significant threat to those grappling with cystic fibrosis. Their focus lies on bacteriophages, viruses that infect bacteria, and their profound impact on immune responses during infections.
The research spotlights a specific bacteriophage, Pf4, known to infect P. aeruginosa. Unlike lytic phages that obliterate their bacterial hosts, Pf4 takes a different temperate approach. While the study (done by the same group of scientists) had previously established Pf4’s influence on the innate immune response, the precise mechanisms had remained elusive until now.
The recent findings uncover Pf4’s distinctive interaction with the immune system. As a single-stranded DNA phage, Pf4 doesn’t induce bacterial lysis. Instead, it triggers unconventional responses in human and murine immune cells, fostering the persistence of bacterial infections.
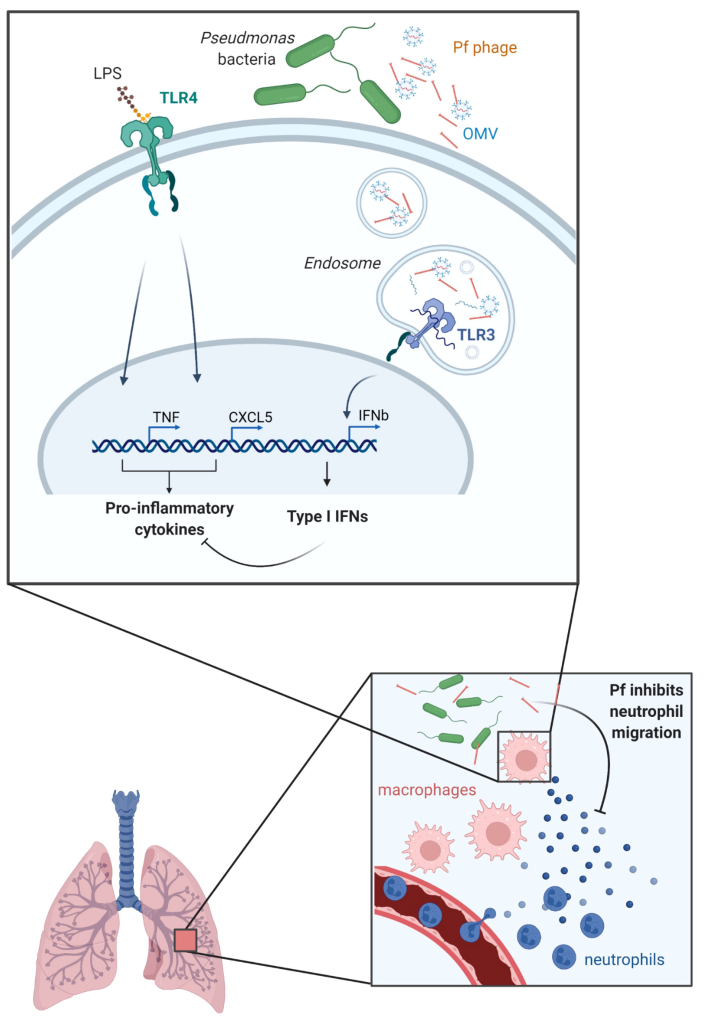
Pf4 is revealed to attach itself to outer membrane vesicles (OMVs) produced by P. aeruginosa. These vesicles are pivotal in the bacterium’s interactions with the host. Upon internalization by human cells, Pf4, coupled with attached OMVs, initiates the formation of endosomal vesicles.
Within these vesicles, short RNAs within the OMVs assume specific structures, setting off a unique immune response known as TLR3-dependent type I interferon production. This response hampers the production of antibacterial cytokines and chemokines, thereby affecting the body’s ability to combat bacterial infections.
The study posits that Pf4, in its interaction with OMVs, curtails inflammatory responses to bacterial endotoxins. Specifically, it suppresses the production of CXCL5, a molecule critical for the chemotaxis of neutrophils—the immune cells tasked with moving toward infection sites. This suggests a compromised ability of the body to attract neutrophils to effectively combat the infection.
In a mouse model mimicking acute pneumonia, mice treated with Pf4 associated with OMVs exhibited significantly fewer neutrophils in their lungs compared to those treated solely with purified Pf4. These observed changes in the immune response were not merely theoretical but had tangible effects on the ability of immune cells to induce neutrophil migration.
These findings present a paradigm shift in our understanding of how Pf4 and potentially other temperate phages, by altering innate immune responses to bacterial endotoxins and OMVs, have the potential to mitigate inflammation at sites of bacterial colonization or infection. The implications of this discovery extend to the realms of cystic fibrosis and beyond, offering new avenues for comprehending and treating complex bacterial infections.
For more information please read the full article here: Pennetzdorfer, N., Popescu, M. C., Haddock, N. L., Dupuy, F., Kaber, G., Hargil, A., … & Bollyky, P. L. (2023). Bacterial outer membrane vesicles bound to bacteriophages modulate neutrophil responses to bacterial infection. Frontiers in Cellular and Infection Microbiology, 13.
Leave a Reply
You must be logged in to post a comment.