The ever-increasing threat to the world’s healthcare system and the constant need to find a replacement for existing antimicrobials have led to the development of antibiotic alternatives and complements. Among the promising alternatives are phage and their derivative products, including whole virions and enzymes derived from the virus.
Most of the time, when mentioning phage therapy, most people associate it with having complete functioning virions that will be applied. However, it has been known that even phages lacking some of the parts may be able to fight bacteria. But what is a bit strange and interesting is that some bacteria can produce these kinds of “defective phages” to kill closely related competitors in the environment. These particles lack the head section and instead have a tail-like structure that allows them to recognise and bind to specific receptors on the surface of target bacteria. These “defective particles” are called tailocins, just like their “friend” assume similar functions as bacteriocins, although in a different form.
Tailocins, also known as phage-tail-like bacteriocins, are highly effective against bacteria and they remain specific just like their look-alike “phages”. Structurally resembling phage tails, tailocins can be produced through phage engineering and other methods by removing the capsid (head) section.
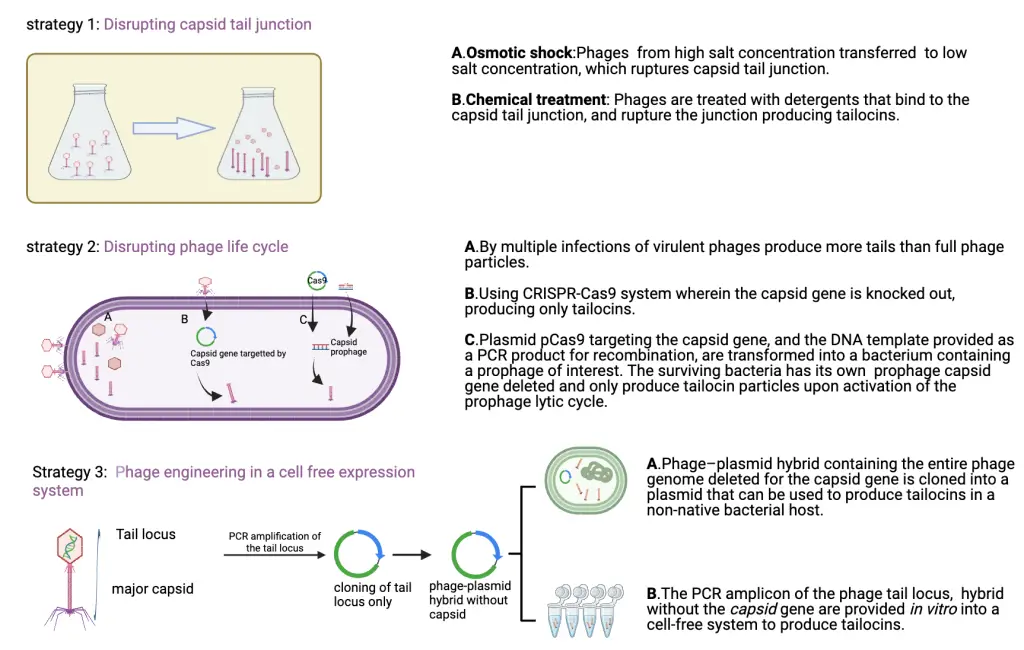
Methods used to make tailocins
To create phages without the capsid (tailocins), several strategies can be employed. The first strategy involves rupturing the phage capsid-tail junction using physical and chemical treatments, resulting in a tail particle that retains its killing activity. The second strategy entails infecting bacteria with multiple phages, leading to the production of a cocktail of tail structures (i.e., tailocins) and fully assembled phage particles. These particles can then be sorted by precipitation and/or sedimentation. The third strategy is to utilize phage engineering by deleting/chopping the capsid genes, hence resulting in a headless phage particle.
Physical and or chemical treatment of phages
To evaluate each strategy in detail, the first one being the physical and or chemical treatment of phages. This method utilises osmotic shock as a physical treatment, which induces the release of genetic material, producing empty ghost-headed phage particles. These ghost phages then kill their bacterial host,s releasing all the cell contents. When this was tested on virulent phages, with their broad host range, this method of osmotic shock did not render them inactive, indicating that osmotic shock is not the best option to produce tailocins. Utilising any natural or synthetic chemical that binds to the phage capsid tail junction as a chemical treatment strategy to inactivate the phages was studied. It is observed that not all phages were rendered inactive, and not all phages produced active tailocins. This concludes that individual phages need to be studied for an effective physical and chemical treatment method to produce desirable tailocins.
Multiple phage infections
The next method wherein multiple phage infections of virulent phages produce multiple whole phages, and also phages with only tails. Those with only tails can be considered as tailocins. However, it remains to be tested if these phages retain their killing ability. Genetic engineering of a virulent phage into a tailocin is carried out using a double-plasmid clustered palindromic repeats (CRISPR-Cas9) system. It remains to be tested if such tailocins would retain their killing activity.
Genetic engineering
In the third method, structural and genetic similarity between the temperate phages and the tailocins is explored. Phage engineering can be employed to remove the genes necessary for capsid and only tail genes can be retained. Such prophages, when propagated in the same bacterial host, lead to the production of a cocktail of tailocins which in turn will bind to different receptors. This broad range of receptor binding will help in identifying potential prophages capable of producing tailocins. This immensely increases the availability of tailocins and expands the repertoire of targeted bacteria.
Phages are self-assembly nanomachines. Therefore, if produced in vitro, their components can auto-assemble if given in the right conditions. Cell-free expression systems, which simulate the transcription and translation capabilities of cells in vitro, have been used so far, for different applications, such as the production of high-yield proteins and in synthetic biology. Recently, they proved effective in producing full phage particles with therapeutic potential applications. Therefore, cell-free extracts provided with DNA from phage genomes lacking capsid genes, a tailocin-plasmid, or a DNA fragment containing the genes for the phage tail synthesis could produce tailocins. Cell-free expression systems may need to be adapted depending on the phage host, as some would require specific factors to initiate transcription. Various parameters can impact the performance of such cell-free systems.
Regardless of the method used to make tailocins, preparations must be purified to remove toxic substances (e.g., endotoxins, bacterial membrane), and their stability must be assessed for clinical safety for therapeutic applications. This can be achieved using similar methods developed for bacteriophage therapy. To reduce the burden on currently available antimicrobials to combat the resistance, alternative therapeutic options are being explored all around the world. In this scenario, with the potential of phage therapy, tailocins, as novel antimicrobials against antibiotic-resistant pathogenic bacteria, will soon be considered a sustainable alternative to current antibiotics.
More reading on scientific articles here
Cover photo credit: iLexx/iStock and Aliyah Kovner/Berkeley Lab