Scientists from the Weizmann Institute of Science in Israel and the Helmholtz Centre for Infection Research in Germany have published their recent research. In their study, they examined the bacterial immune system and discovered a mechanism by which bacteria attach minute proteins to the tails of phages. Interestingly, these proteins resemble those used by the human immune system to stop viral infection. The proteins are conjugated to both viral and host molecules to prevent infection. This defense system, which includes a ubiquitin-like protein, ubiquitin-conjugating enzymes (E1 and E2), and a deubiquitinase, is initiated during phage infections.
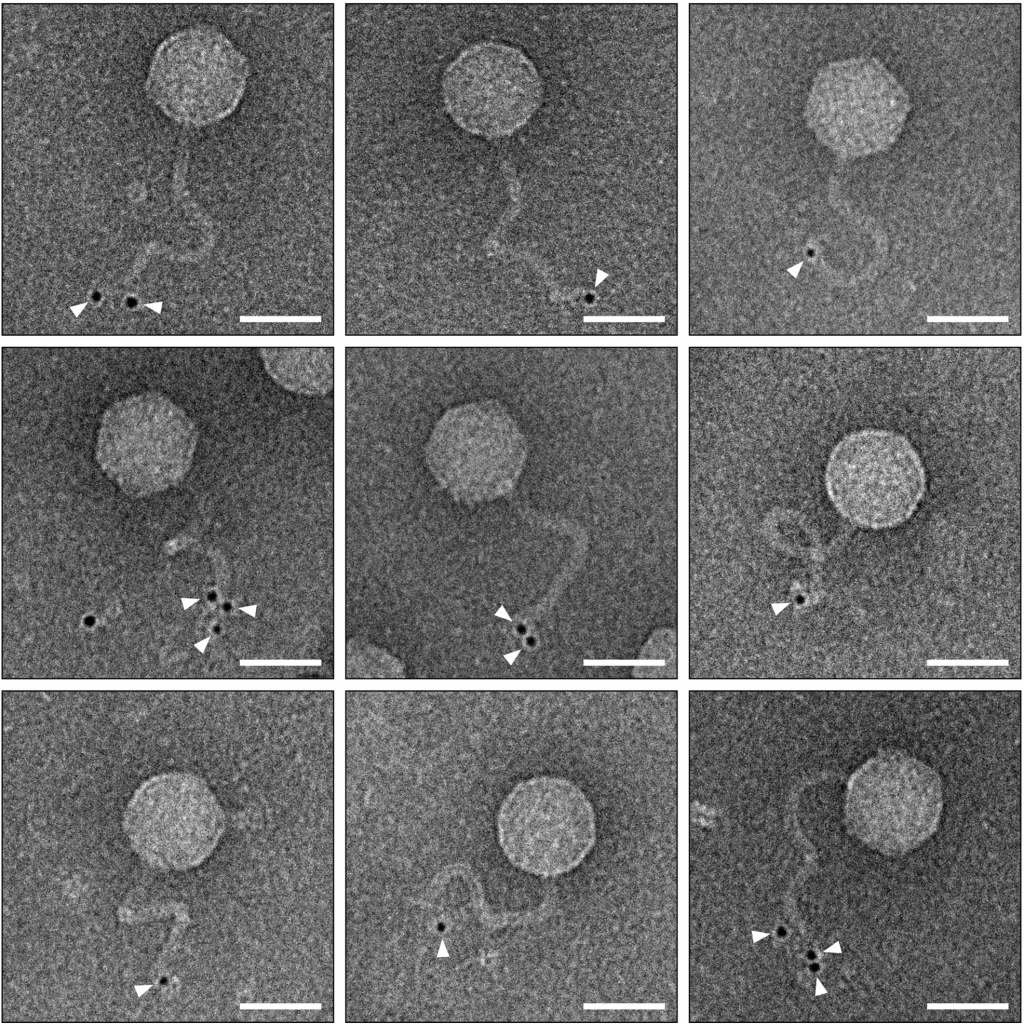
During such infections, the bacterial system conjugates the ubiquitin-like protein specifically to the phage’s central tail fiber, which is crucial for tail assembly and host recognition. As a result, the infected cells produce a mix of incomplete, tailless phage particles and fully assembled ones whose central tail fibers are blocked by the ubiquitin-like proteins. These modified phages have severely diminished infectivity, protecting the bacterial population from further viral spread. This newly discovered bacterial defense demonstrates that ubiquitin-like protein conjugation is a conserved antiviral mechanism across various forms of life.
Recent studies also highlight the fascinating structure of phages—viruses that attack bacteria—which have a head containing genetic material and a tail used to identify and infect host cells. Once inside the bacterial cell, the phage hijacks the host’s machinery to replicate itself. However, the bacterial immune system recently identified uses ubiquitin-like proteins to stop this process.

This bacterial immune system works by attaching the small protein to the phage’s tail, effectively neutralizing its ability to infect other cells. Notably, the components of this system resemble human immune mechanisms, providing insights into how our own immune system may have evolved.
For decades, research into bacterial defense mechanisms yielded few discoveries. CRISPR-Cas9 is one of the most famous examples, revolutionizing gene editing. Yet, recent discoveries of over 150 bacterial immune systems suggest a plethora of defense strategies, including systems involving ubiquitin-like proteins. These findings were made possible by exploring gene clusters in bacterial genomes known as “defense islands,” which tend to harbor genes related to immune function.
A key discovery from this new research was that bacteria employing this immune system still allow viral duplication, but the resulting viruses are “sterile”—they cannot infect new bacterial cells. This is due to the ubiquitin-like protein binding to the viral tail, preventing it from functioning properly. Scientists used advanced microscopy techniques to visualize how this mechanism works at a molecular level, showing the ubiquitin-like protein attaching to the tail tip, obstructing further infection.
This research suggests that similar mechanisms may exist in human immunity. While human viruses do not have tails like phages, disrupting critical viral proteins could be a strategy used by immune proteins like ISG15, which is known to play a role in defending against viruses such as influenza and HIV. By studying bacterial systems, researchers may uncover broader principles that govern how ubiquitin-like proteins function in human immunity.
This newly discovered bacterial system is one of many that scientists are now exploring, each potentially offering new insights into antiviral defense strategies across different domains of life. Understanding these mechanisms could inspire novel treatments and technologies, especially in areas like virology and gene editing.
You can access their article here Hör, J., Wolf, S.G. & Sorek, R. Bacteria conjugate ubiquitin-like proteins to interfere with phage assembly. Nature 631, 850–856 (2024). https://doi.org/10.1038/s41586-024-07616-5
Leave a Reply
You must be logged in to post a comment.