Wound healing is a complex process that involves a lot of work from our cells. Infections from fractures (broken bones) often involve biofilms—layers of bacteria that make infections harder to treat and resistant to antibiotics. Clinicians face challenges in treating patients with fracture-related infections (FRI), especially when dealing with bacteria that have generated resistance against commonly used drugs that kill them. However, promising global research shows that phage therapy—using viruses that target bacteria—can be effective. One promising approach is the use of hydrogels to deliver phages directly to wound infections, showing positive results in combating these tough bacterial infections.
In musculoskeletal infections, as in the case of infection in fracture wounds, direct application of phages has its limitations. As a fact, the phages can not be applied to the deeper tissues of the wound site. An alternative approach is hydrogels loaded with phages which offer a slow and sustained release of phages at the site.
A study was conducted to develop a mix of phage cocktail and antibiotics, targeting MRSA (Methicillin resistant Staphylococcus aureus, a Gram-Positive Bacteria), packed in hydrogels. Hydrogel has been used as a material for wound dressings and drug depots for prolonged drug release. Hydrogels are now being engineered to incorporate phages for the treatment of wound infection in this study.
The chosen antibiotic was Vancomycin as it is often reserved as the “drug of last resort”, used only after treatment with other antibiotics has failed. Vancomycin is active against most strains of gram-positive bacteria both in vitro and in clinical cases.
A biofilm was lab-created on glass beads for the in-vitro study. Anti-biofilm effects of phage cocktails and vancomycin at different MIC(Minimum inhibitory concentrations) were evaluated. The release kinetics over a time period were studied individually and in combination using RT-qPCR.
It was observed, that in the co-delivery hydrogel, the addition of vancomycin did not affect the release kinetics of phage cocktail over time. However, the addition of phages led to a decrease in the amount of vancomycin released over time compared to conditions where vancomycin alone was delivered via the hydrogel.
Statistical analyses were performed on the data for different hydrogel concentrations, but no significant differences were found. This demonstrates that the observed trends in phage and vancomycin release and stability are consistent across the conditions tested. The results showed that the combination of phage cocktails and vancomycin had the highest cell count reduction.
The in-vivo study on mice was carried out to check the efficacy of co-delivery hydrogel against MRSA FRI. Bacterial load was sampled and quantified from the soft tissues, femur, and implants at euthanasia. The same was done from all the locations in the group that received phage therapy. The serum was also analyzed in both cases.
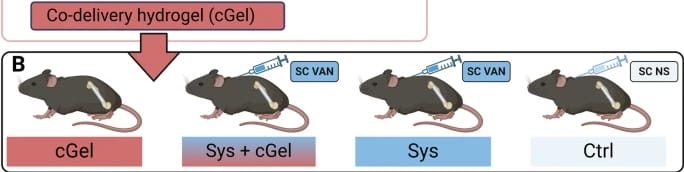
The mice were randomly assigned to one of four treatment groups. cGel group: a single dose of locally administered co-delivery hydrogel; cGel+Sys group: a single dose of locally administered co-delivery hydrogel and systemic subcutaneous vancomycin; Sys group: subcutaneous vancomycin administration; and Ctrl group: only received 0.9% NaCl solution.
The isolated strains were tested with phages alone and phages combined with systemic vancomycin were analyzed as well. Results indicated bacterial clearance in the group tested with phages and systemic vancomycin than when treated with phages alone. In vivo, the co-delivery hydrogel combined with vancomycin significantly reduced the bacterial load compared to systemic vancomycin alone and untreated controls.
This suggests that Phage therapy combined with vancomycin increased bacteria susceptibility to phages. Taken together, these outcomes confirm the value of a co-delivery hydrogel system to decrease the bacterial load and increase phage persistence in vivo.
Prolonged antibiotic therapy, repeated surgical interventions in FRI, and biofilms act as diffusion barriers and play a major role in antimicrobial resistance. Introducing optimal delivery systems is critical to achieve better antibacterial efficacy. Phage therapy has been shown to effectively tackle such infections along with antibiotics acting as an adjunct.
The use of phages as therapeutic agents in vivo is promising. However, it raises concerns about the host’s immune response potentially neutralizing the phages. Data in the scientific literature is rather limited regarding this and more research is the need of the hour.
This article is based on the research paper published in Nature, the link to which is, https://www.nature.com/articles/s41522-024-00552-2
Leave a Reply
You must be logged in to post a comment.