A urinary tract infection (UTI) is an infection that affects any part of the urinary system, which includes the kidneys, ureters, bladder, and urethra. Most UTIs occur in the lower urinary tract, specifically in the bladder and urethra. In 2019, a global study recorded 404 million UTI cases. Another study found that about 12% of patients with a catheter in place for 30 days develop a catheter-associated UTI (CAUTI).
Proteus mirabilis (P. mirabilis) is a common bacteria often found in patients using catheters or drainage bags. It plays a significant role in catheter-associated infections. This bacterium produces an enzyme called urease, which breaks down urea in the urine. This process raises the urine’s pH, making it more alkaline. The higher pH leads to the formation of crystals that can block the catheter. These crystals also encourage the development of biofilms, which are protective layers formed by bacteria. The biofilms make it harder to treat the infection and further worsen the catheter blockage, creating a cycle of obstruction and infection.
Scientists have been exploring effective ways to eliminate biofilms, which are a major challenge in treating infections. Although antibiotics seem ideal due to their broad-spectrum activity, they often fail because the biofilm structure acts as a barrier, preventing antibiotics from reaching the bacteria, and many bacteria within biofilms enter a dormant state, reducing their susceptibility. Biofilm formation often starts with a primary colonizer, such as P. mirabilis in catheter-associated infections, and targeting this colonizer can help prevent biofilm development. Researchers are now investigating innovative approaches like combining plasma-activated water (PAW) with phage therapy. PAW contains reactive species that disrupt bacterial cells, while phages are highly specific viruses that target bacteria. By designing phages to specifically attack P. mirabilis, scientists aim to disrupt biofilm formation at its source, offering a more precise and effective alternative to antibiotics.
what is Plasma-activated water (PAW)?
Plasma-activated water (PAW) is water that has been treated with a special type of energy called plasma. Plasma is a high-energy state of matter, like the one found in lightning or neon signs, and it creates reactive particles when it interacts with air or water. When water is exposed to plasma, it becomes filled with these reactive particles, such as reactive oxygen and nitrogen species, which can kill bacteria and other harmful microorganisms. PAW is being studied as a safe and effective way to fight infections, including those caused by biofilms because it can break down bacterial cells without using harsh chemicals or antibiotics.
Combination treatment of PAW and Phage against Biofilm cells
In a recent study, scientists from Queen’s University Belfast and University College Dublin explored the combined effects of bacteriophages and plasma-activated water (PAW). They examined how variations in plasma discharge parameters, such as design and power, impact the antimicrobial effectiveness of PAW. The research also focused on understanding the interactions between phages and PAW when applied to biofilms—whether these interactions are synergistic, enhancing biofilm eradication, or antagonistic, reducing their effectiveness. By addressing these questions, the study aims to reveal the potential of using bacteriophages and PAW together to overcome the defense mechanisms of P. mirabilis biofilms.
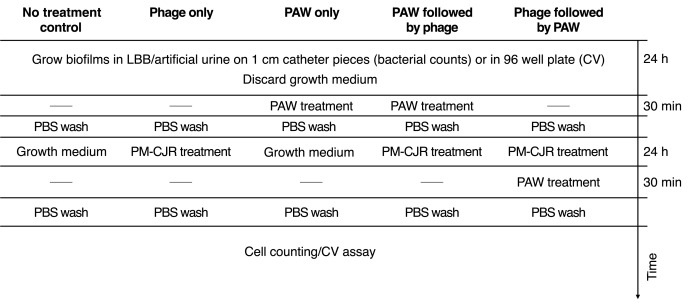
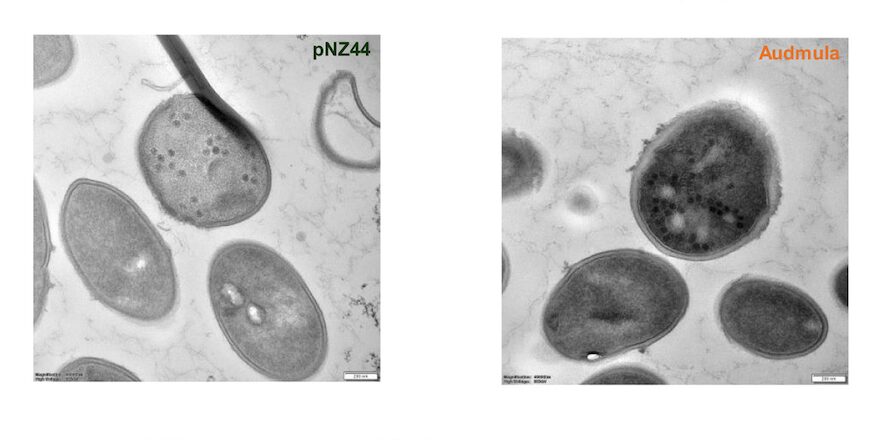
The team assessed the effectiveness of combining Spark 30 PAW and phage treatment against P. mirabilis biofilms by testing four treatment groups: phage-only, PAW-only, PAW followed by phage, and phage followed by PAW. The treatments were applied to P. mirabilis BB2000 biofilms grown in artificial urine for 24 hours on urinary catheter segments, with viable cell counts and crystal violet assays conducted. The study also included tests to measure reactive oxygen and nitrogen species (ROS/RNS), pH changes, susceptibility of both planktonic and biofilm cultures, and the stability of bacteriophages in PAW, evaluating the combined effects of PAW and phage treatment on biofilm cells.
Results
Quantitative analysis of PAW treated with Spark and Glow discharge revealed the differential generation. The results showed that Spark 30 PAW was more effective at killing bacteria and keeping phages stable compared to Glow discharge PAW. In Spark-treated PAW, the amount of hydrogen peroxide (a reactive substance) increased, while no nitrite was found. Nitrate levels also rose, and the pH dropped from 7.1 to 1.67 as the treatment went on. In Glow-treated PAW, there was no hydrogen peroxide, but nitrite and nitrate levels still increased, and the pH dropped from 7 to 1.90. The biggest changes happened in the first 10 minutes of treatment, but continued treatment for up to 30 minutes led to even more reactive substances and a further decrease in pH.
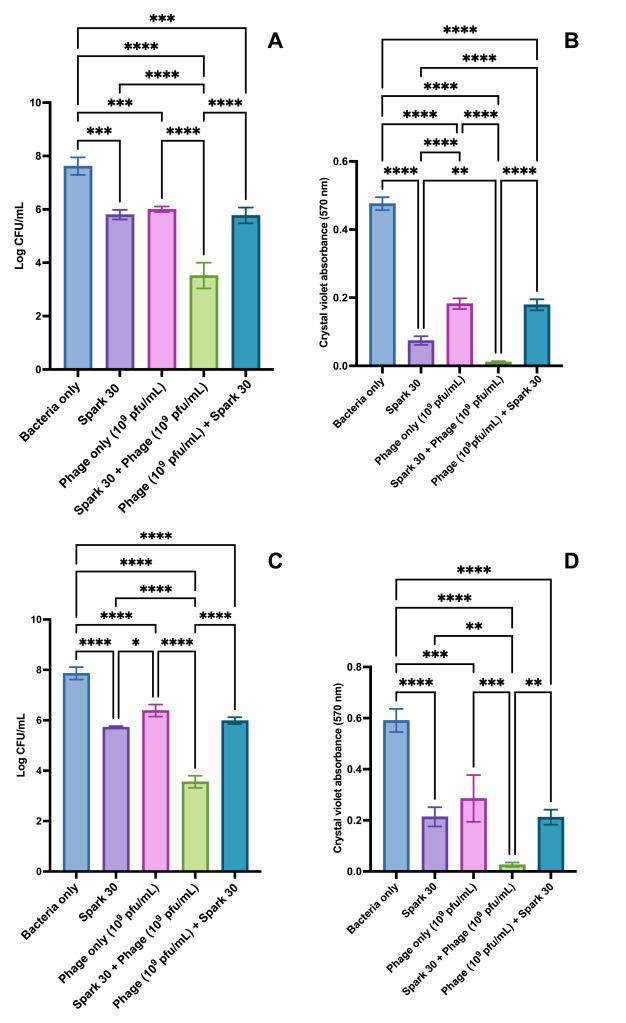
When the combination of phages and PAW was tested, PAW treated for 30 minutes completely killed the bacteria within 10 minutes. However, when tested on biofilms (bacteria clumped together), PAW did not work as well as it did on free-floating bacteria. When both phages and PAW were used together on biofilms in artificial urine, there was a large reduction in bacteria after 24 hours. However, when used separately, phages or PAW only reduced bacteria by a smaller amount. There was no noticeable difference between using phages alone and using Spark 30 PAW alone.
For all those difficult-to-treat biofilms, a new innovative strategy is required to treat the severely affected patient cases, especially in CAUTI. Working with the phage therapy has been one of them. Further to expedite the process, using PAW showed promising results through this study.
Plasma medicine is an emerging field, its applications and therapeutic effects are being studied across clinical medicine, life sciences, and cancer treatments. Though plasma therapy is yet to be implemented on a larger scale, studies have shown promising results. While most research so far has been on phage-antibiotic combination treatments, this study is an example of that hope where phages in combination with plasma can be developed as another novel approach to combat multi-drug resistant infections.
This article is written based on a study, link to the study is https://www.sciencedirect.com/science/article/pii/S2590207524000558

Leave a Reply
You must be logged in to post a comment.