Scientists have developed a novel method to significantly boost the effectiveness of bacteriophage therapy, a promising alternative to antibiotics for treating bacterial infections. By encapsulating phage-infected bacteria in a protective polymer nanocoating, researchers preserved the vitality of phages, leading to superior treatment outcomes in animal models of intestinal infection and associated arthritis. The findings, published in a recent issue of Nature, could revolutionize the fight against antibiotic-resistant pathogens.
The Challenge of Phage Vitality
Bacteriophages, viruses that infect and kill bacteria, have gained attention as precision tools against antibiotic-resistant infections. However, their effectiveness is often compromised when phages are released from host bacteria or exposed to harsh environments during production and delivery. This degradation reduces their ability to target and destroy pathogens, a major hurdle in clinical applications.
A Nano-Shield for Phages
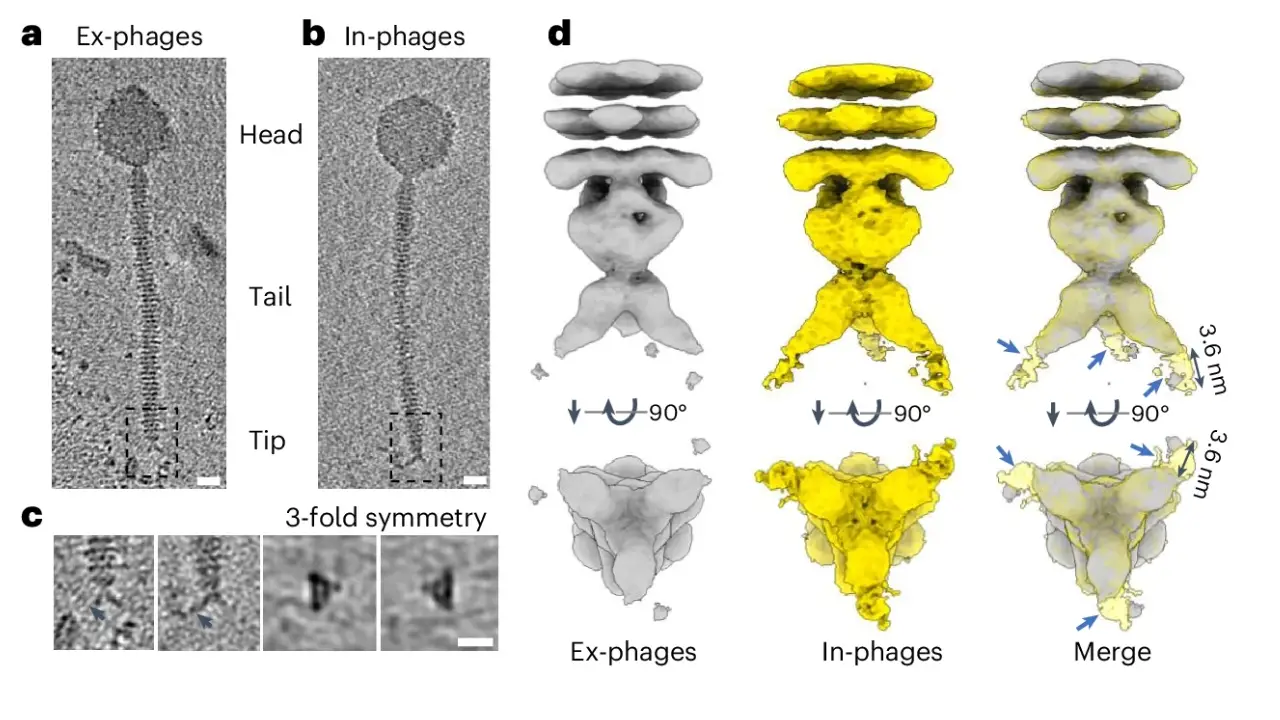
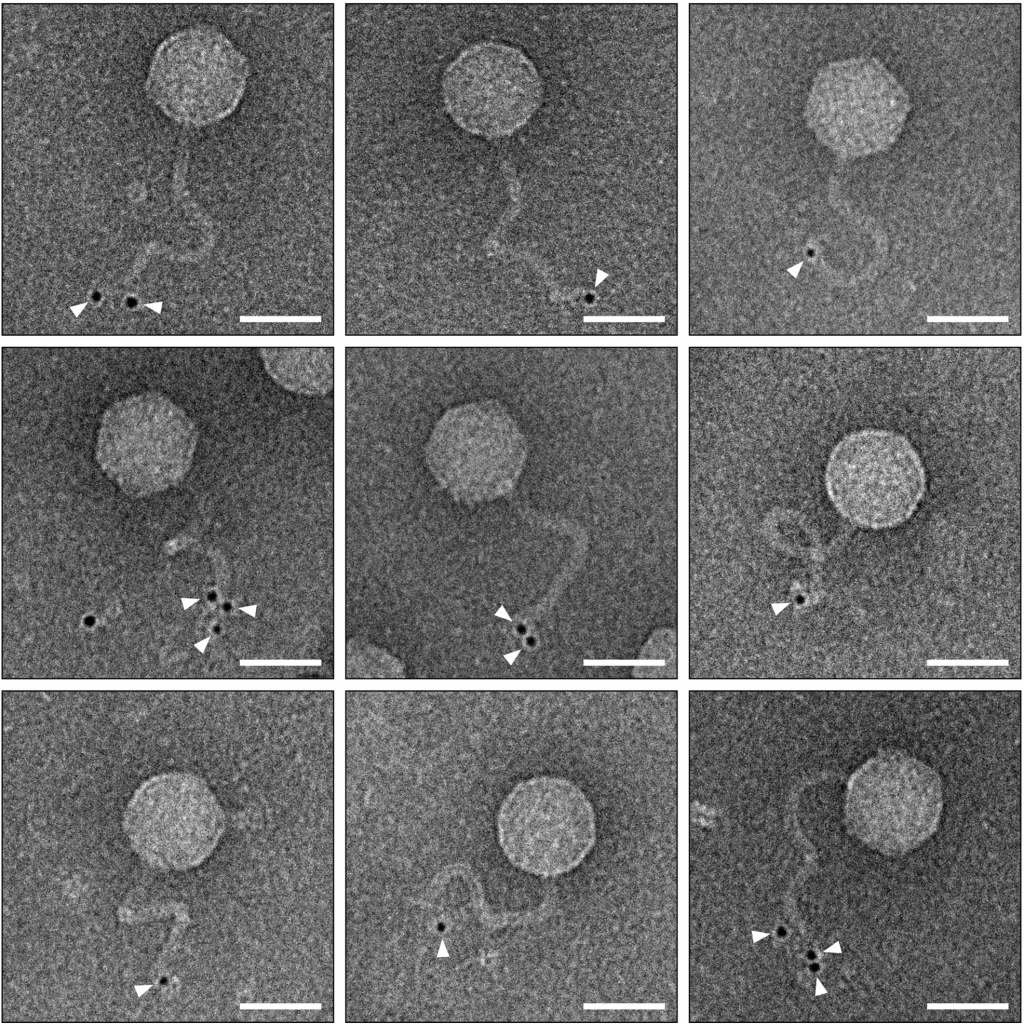
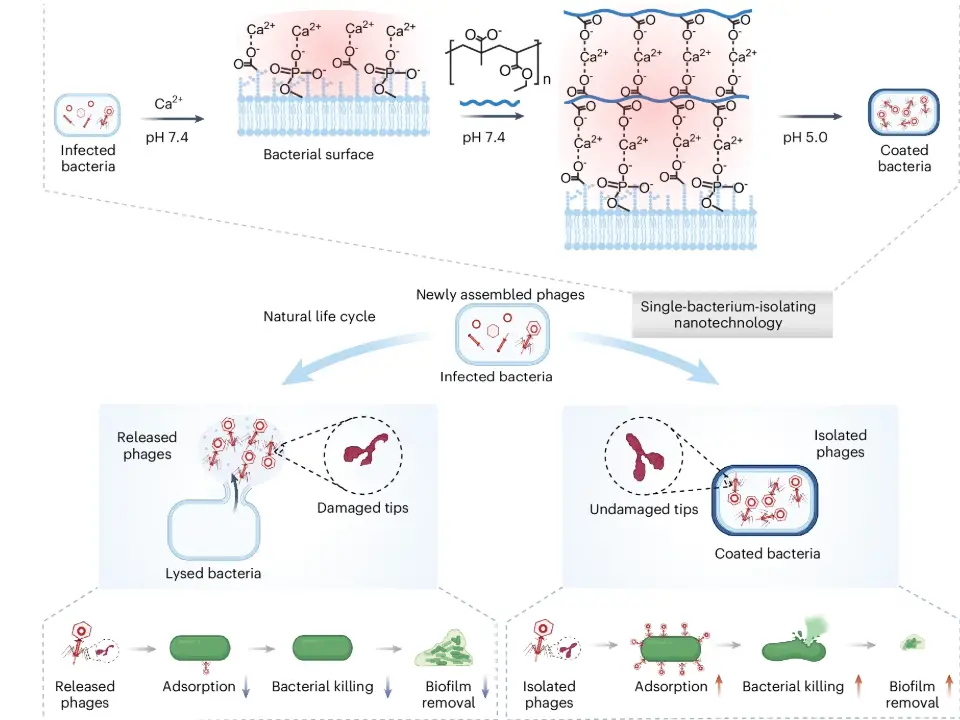
The research team addressed this issue by wrapping individual phage-infected bacteria in a polymer nanocoating. This shield preserves the phages’ microenvironment, protecting them from external stressors. When the bacteria lyse (burst), the newly formed “In-phages” remain intact within the coating, unlike conventionally released “Ex-phages,” which suffer protein damage.
Key Findings
- Enhanced Potency: In-phages demonstrated 3–18 times higher vitality than Ex-phages in various media and temperatures, maintaining their ability to amplify and attack bacteria.
- Biofilm Eradication: In-phages penetrated and dismantled bacterial biofilms, a major source of chronic infections up to three times more effectively.
- Targeted Delivery: Coated with a pH-sensitive polymer, the phages survived stomach acid and released intact in the intestines. In mice with Salmonella-induced enteritis, oral In-phage treatment reduced gut bacterial counts by 90% compared to conventional phages.
- Arthritis Relief: The same treatment alleviated reactive arthritis linked to intestinal infection, reducing inflammatory markers and joint damage by preserving gut barrier integrity.
Mechanism Unveiled
Advanced imaging and proteomics revealed that Ex-phages lose critical tail proteins (e.g., Carrier domain-containing protein) due to environmental exposure, impairing their ability to bind and lyse bacteria. In-phages retained these proteins, explaining their superior performance.
Safety and Future Implications
The treatment showed no adverse effects in mice, with normal organ function and blood parameters. This approach not only enhances phage therapy’s efficacy but also enables oral administration previously hindered by gastric acid degradation.
“By maintaining phage vitality until they reach infection sites, we’ve overcome a major translational barrier,” the authors stated. As antibiotic resistance escalates globally, this innovation paves the way for more reliable phage-based treatments against stubborn infections, from gut pathogens to biofilm-associated diseases.
The study underscores the potential of nanotechnology to breathe new life into phage therapy, offering hope in the post-antibiotic era.
Reference
Lin, S., Xie, G., He, J. et al. Enhancing phage therapy by coating single bacteriophage-infected bacteria with polymer to preserve phage vitality. Nat. Biomed. Eng (2025). https://doi.org/10.1038/s41551-025-01354-3