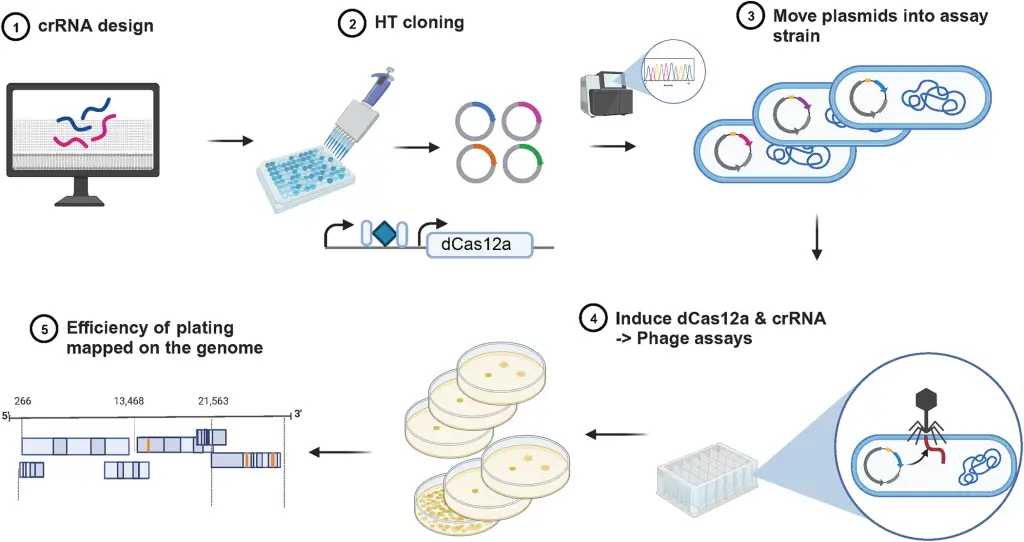
Two phage-based vaccine candidates for avian influenza (H5N1) developed by Cytophage are now entering preclinical trials. Cytophages’ innovative phage technology uses distinct antigen presentation strategies to enhance immune response and cross-strain protection. The preclinical trial will assess efficacy, immunogenicity, and optimal dosing in animal models.
To trigger an immune response, the two vaccine candidates are using filamentous phages to display influenza antigens. “Traditional vaccine models take too long to respond to emerging influenza threats,” said Natasha Theriault, Project lead of the vaccines unit at Cytophage. This new phage-based vaccine model will allow the deployment of a targeted vaccine at a higher speed and with better efficacy.
If successful, Cytophage’s phage-based vaccine could play a crucial role in cost-effective pandemic preparedness and an adaptable alternative to existing vaccine technologies. Cytophage Technologies is a biotechnology company dedicated to phage research, product development, manufacturing and commercialization in Canada.
H5N1 virus has wreaked havoc on the poultry population, leading to the culling of chickens across the globe. The virus is also detected in cattle and has crossed into human infections.