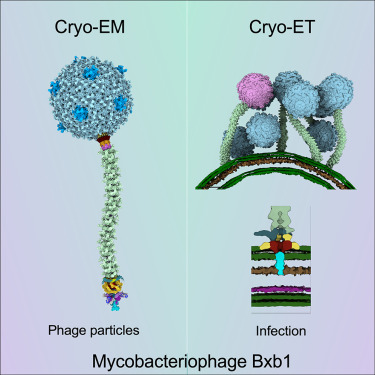
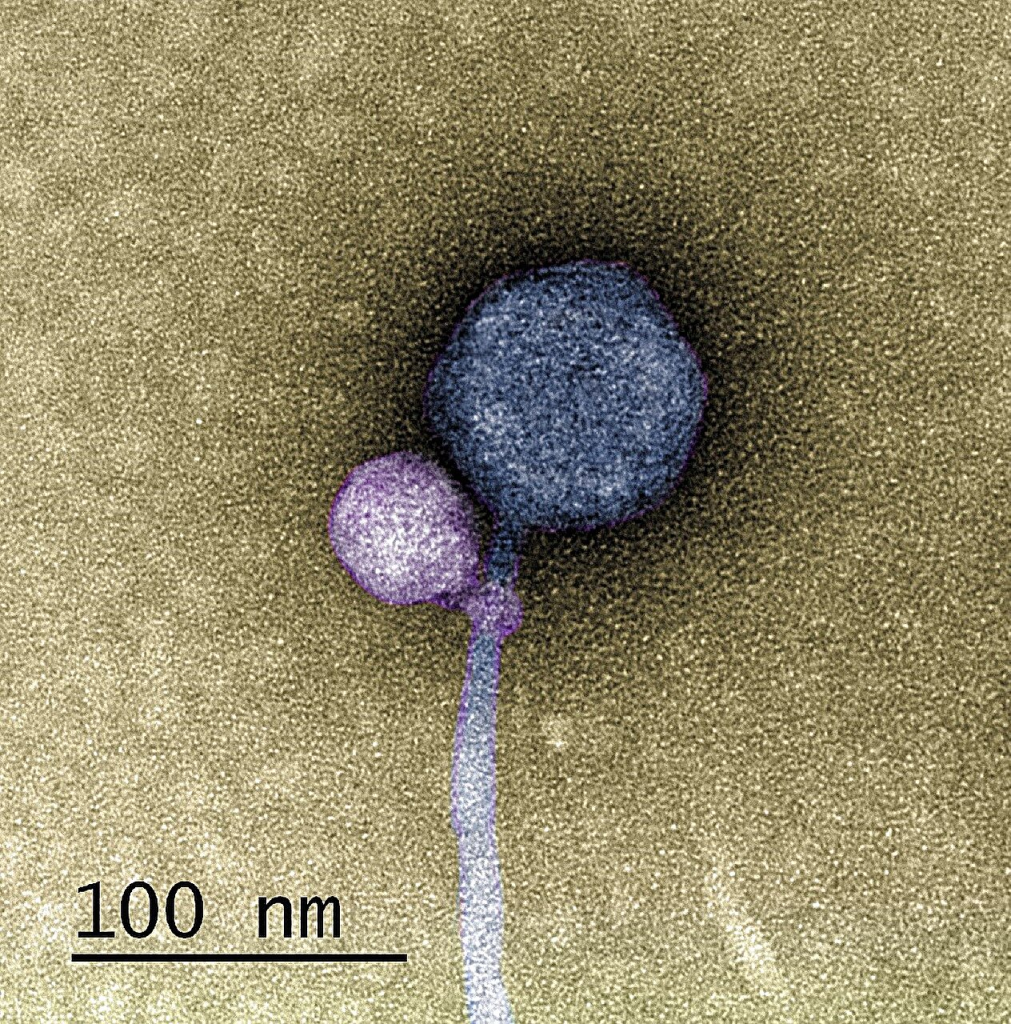
Phage research is advancing with the progression in technologies and instrumentation. With all these come new insights into phage structure beyond what already exists. Recently, in the Journal Cell, researchers from the University of Pittsburgh published various insightful images showcasing the capsid structure in more detail using cryo-electron microscopy(Cryo-EM). Researchers utilized the mycobacteriophage Bxb1, a well-characterized virus of Mycobacterium smegmatis with double-stranded DNA and a long tail, with an image resolution at the atomic level.
Utilizing cryo-EM helped researchers identify the conformational changes when the mycobacteriophage Bxb1 approaches the Mycobacterium. The complete structure and atomic model of the phage Bxb1, including the subunits in the capsid and the tail tip, were elucidated. These structural analyses suggest that several Bxb1 proteins likely contribute to host specificity during infection. Cryo-electron tomography also revealed the structural transitions occurring, enabling the phage to transfer its DNA into the cytoplasm.
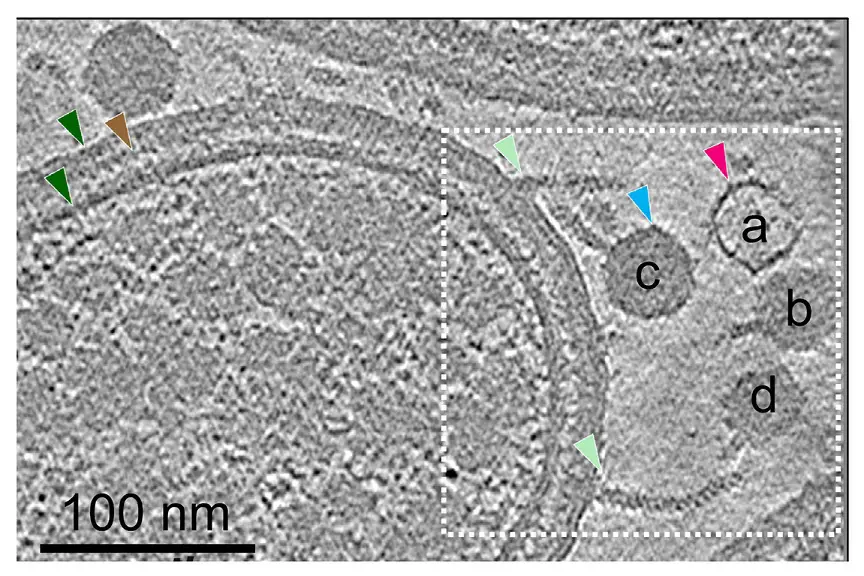
This becomes a more significant advancement because phages have to traverse the tough mycolic acid outer membrane. “Elucidation of determinants of phage specificity and the mechanisms of DNA transfer to the cytoplasm are critical for advancing the potential of mycobacteriophages for the therapy of tuberculosis and non-tuberculosis mycobacterium (NTM) infection.
Such advances in cryo-EM have led researchers to whole phage structural characterization at a near-atomic level, and virions of several other hosts have been described, including podophages, myophages, siphophages, and direct 3D visualization of host-bound phages.
The tail and tail tips play crucial roles in host recognition, binding, and DNA transfer. These findings will also advance mycobacteriophage therapies for tuberculosis and NTM infections.
Such technological advances will eventually pave the way for improved resolution of phages and their structures, making phage engineering easy.
Read the full article here https://www.cell.com/cell/fulltext/S0092-8674(25)00345-9