Bacteriophages are viruses that affect bacteria and not plants, humans,s or any other mammals; due to their specificity, it poses no harm to other creatures except that specific bacteria host. To determine whether which laboratory can handle which bacteriophage depends on the host used instead of the phage managed, therefore the determinant is bacteria and not the virus. For example, bacteriophages for Mycobacterium must be handled in higher labs since the host (the deadly bacteria) will be used at one point. Let’s polish our knowledge on bio-safety laboratory levels.
Bio-safety Level 1
Biosafety level one, the lowest level, applies to work with agents that usually pose a minimal potential threat to laboratory workers and the environment and do not consistently cause disease in healthy adults. Research with these agents is generally performed on standard open laboratory benches without special containment equipment. SL 1 labs are not usually isolated from the general building. Training on the specific procedures is given to the lab personnel, supervised by a trained microbiologist or scientist.
Standard microbiology practices are usually enough to protect laboratory workers and other employees in the building. These include mechanical pipetting only (no mouth pipetting allowed), safe sharps handling, avoidance of splashes or aerosols, and decontamination of all work surfaces when work is complete, e.g., daily. Decontamination of spills is done immediately, and all potentially infectious materials are decontaminated before disposal, generally by autoclaving. Standard microbiological practices also require attention to personal hygiene, i.e., hand washing and a prohibition on eating, drinking, or smoking in the lab. Standard laboratory personal protective equipment is generally worn, including eye protection, gloves, and a lab coat or gown. Biohazard signs are posted, and access to the lab is limited whenever infectious agents are present.
Bio-safety Level 2
Biosafety level two would cover work with agents associated with human disease, in other words, pathogenic or infectious organisms posing a moderate hazard. Examples are the equine encephalitis viruses and HIV when performing routine diagnostic procedures or working with clinical specimens. Therefore, because of their potential to cause human disease, great care is used to prevent percutaneous injury (needlesticks, cuts, and other breaches of the skin), ingestion, and mucous membrane exposures in addition to the standard microbiological practices of BSL 1. Contaminated sharps are handled with extreme caution. Use of disposable syringe-needle units and appropriate puncture-resistant sharps containers is mandatory. Direct handling of broken glassware is prohibited, and decontamination of all sharps before disposal is standard practice. The laboratory’s written biosafety manual details any needed immunizations (e.g., hepatitis B vaccine or TB skin testing) and whether serum banking is required for at-risk lab personnel. Access to the lab is more controlled than for BSL 1 facilities. Immunocompromised, immunosuppressed, and other persons with increased risk for infection may be denied admittance at the discretion of the laboratory director.
BSL 2 labs must also provide the next level of barriers, i.e., specialty safety equipment and facilities. Preferably, this is a Class II biosafety cabinet or equivalent containment device for work with agents and an autoclave or other suitable method for decontamination within the lab. A readily available eyewash station is needed. Self-closing lockable doors and biohazard warning signs are also required at all access points.
Bio-safety Level 3
Yellow fever, St. Louis encephalitis, and West Nile virus are examples of agents requiring biosafety level 3 practices and containment. Work with these agents is strictly controlled and registered with all appropriate government agencies. These are indigenous or exotic agents that may cause severe or lethal disease via aerosol transmission, i.e., simple inhalation of particles or droplets. The pathogenicity and communicability of these agents dictate the next level of protective procedures and barriers. Add to all the BSL 2 practices and equipment even more stringent access control and decontamination of all wastes, including lab clothing before laundering, within the lab facility. Baseline serum samples are collected as appropriate from all lab and other at-risk personnel.
More protective primary barriers are used in BSL 3 laboratories, including solid-front wraparound gowns, scrub suits or coveralls made of materials such as
Tyvek® and respirators as necessary. Facility design should incorporate self-closing double-door access separated from general building corridors. The ventilation must provide ducted, directional airflow by drawing air into the lab from clean areas and without recirculation.
Bio-safety Level 4
Agents requiring BSL 4 facilities and practices are hazardous and pose a high risk of life-threatening disease. Examples are the Ebola virus, the Lassa virus, and any agent with unknown risks of pathogenicity and transmission (Laboratory insider listed top ten of the most lethal viruses here). These facilities provide maximum protection and containment. To the BSL 3 practices, we add requirements for complete clothing change before entry, a shower on exit, and decontamination of all materials before leaving the facility.
The BSL 4 laboratory should contain a Class III biological safety cabinet. Still, it may use a Class I or II BSC in combination with a positive-pressure, air-supplied full-body suit. Usually, BSL 4 laboratories are in separate buildings or a totally isolated zone with a dedicated supply and exhaust ventilation. Depending on the agents used, exhaust streams are filtered through high-efficiency particulate air (HEPA) filters.
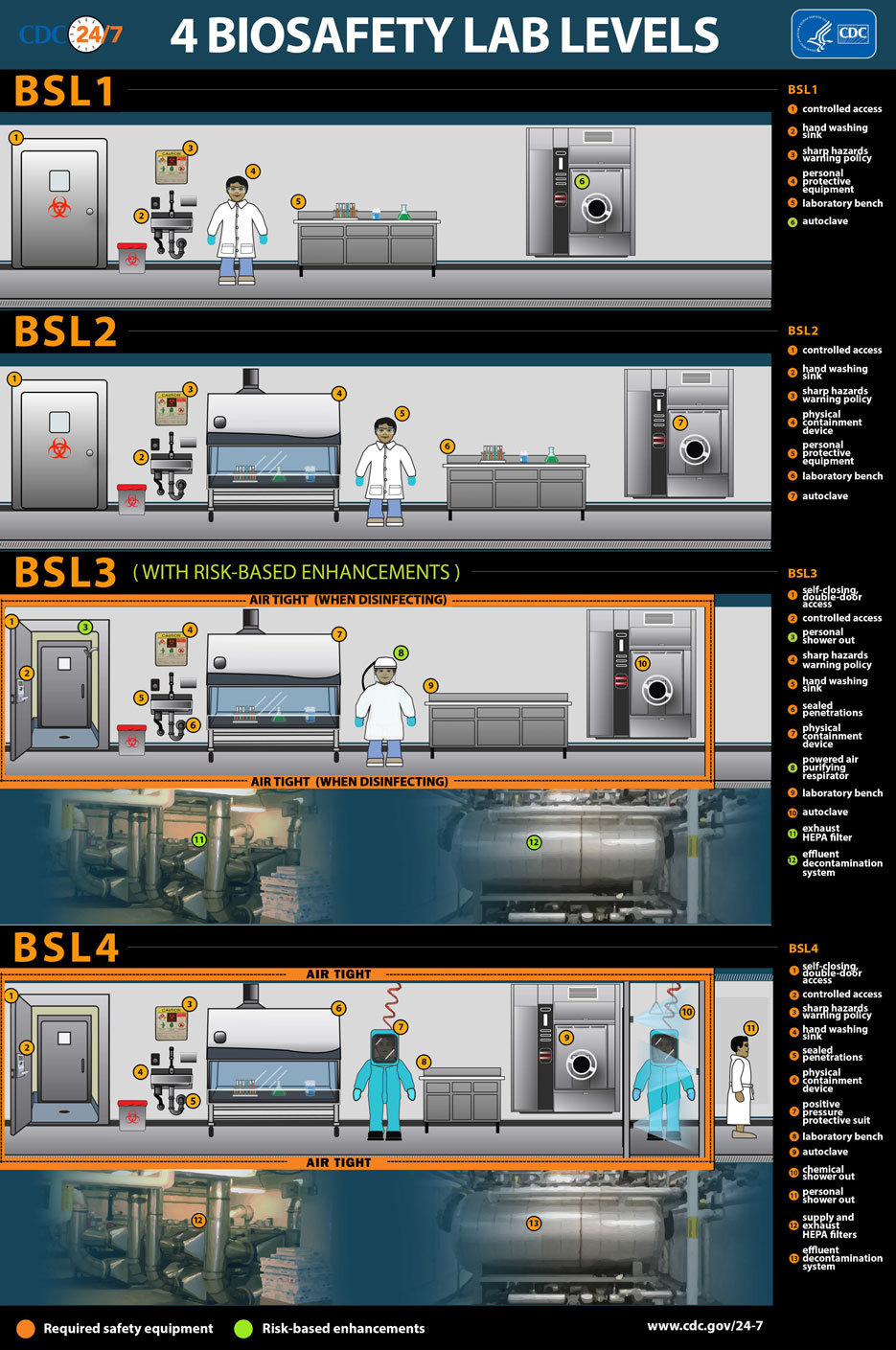 |
Biosafety lab levels by CDC
|