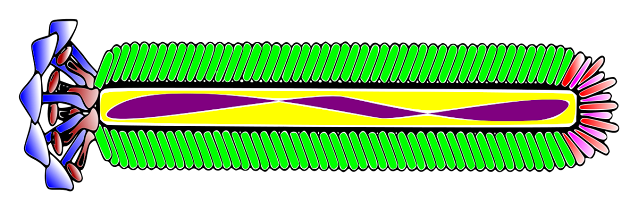
Circular ssDNA in filamentous bacteriophages forms an antiparallel two-stranded helix (similar to A-DNA or B-DNA). Because DNA is circular, the helix ends with two loops. The packaging signal is a hairpin that targets the genome for packaging and initiates filamentous phage assembly. Phosphates along the DNA helix interact with positively charged residues of the major coat protein, resulting in the formation of a helically symmetrical tube that gives the virion its filamentous appearance. The Watson-Crick type of pairing is only maintained for about 25% of nucleotide pairs due to the lack of complementarity (with the exception of a palindromic sequence that forms a hairpin loop – the packaging signal). Some filamentous bacteriophages form a helix with phosphates in the middle and bases pointing outwards.
Structure and genome size
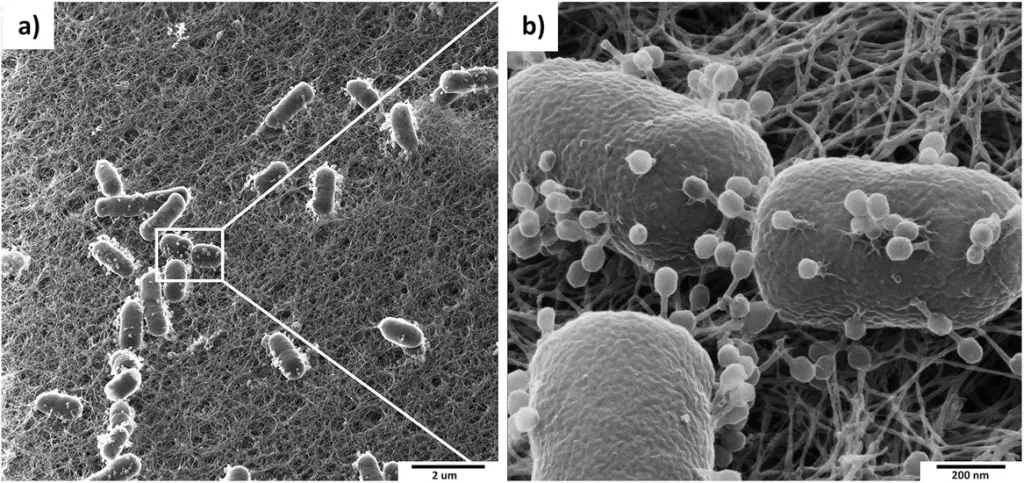
Filamentous phage virions are long, flexible filaments 6-7 nm in diameter and varying in length depending on the length of the packaged DNA. Multiple genomes can be assembled into long filaments that can extend up to 20 mm if the initiation or termination of filament assembly is hampered.
Physical-chemical properties
Resistance to a wide range of pH and temperatures is a common feature of filamentous phage virions. They are nonionic detergent resistant, and some are also ionic detergent resistant. Chloroform sensitivity is shared by all filamentous phages. Filamentous bacteriophage virions do not contain lipids, which is surprising given that all virion proteins are integral inner membrane proteins before assembly. Filamentous bacteriophages behave like liquid crystals at high concentrations and can be aligned in a strong magnetic field. These Pf1 phage properties have been used in nuclear magnetic resonance (NMR) structural studies of other proteins by promoting protein alignment in the magnetic field.
Assembly of the filamentous phage virions (In detail)
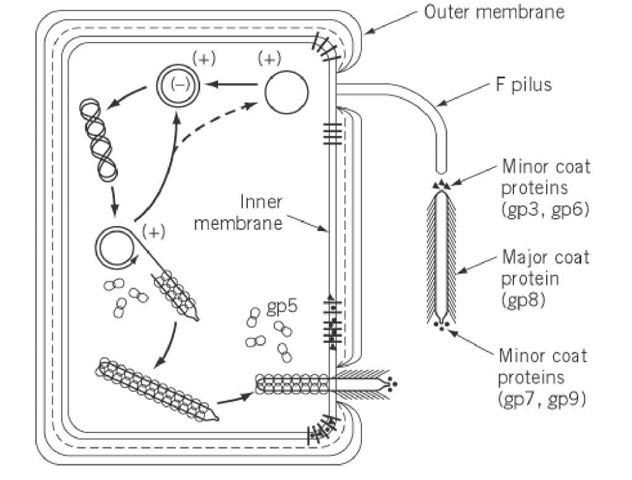
All virion proteins are integral membrane proteins prior to virion assembly. Four of the five virion proteins have a single transmembrane a-helix near the C terminus and a few C-terminal residues in the cytoplasm (pVIII, pIII, pVII, and pIX). Three transmembrane helices are predicted for pVI, the virion’s fifth protein. The major coat protein is directed to the membrane, resulting in Ff infection and pVIII becoming one of the cell’s dominant proteins. This is unusual because the inner membrane protein translocation complex SecYEG usually limits the number of proteins that can be incorporated into the inner membrane. pVIII employs the alternative translocon YidC may aid in overcoming the SecYEG bottleneck, allowing for massive overproduction and membrane targeting.
Filamentous phage assembly is a secretion-assembly process similar to pilus assembly or toxin secretion via dedicated trans-envelope protein secretion systems.’ The packaging signal interacts with two minor proteins, pVII, and pIX, as well as the inner membrane assembly complex (via pI), to initiate assembly. The assembly process then continues by adding major coat protein pVIII subunits until the entire DNA helix is covered. Virion proteins translocate from the inner membrane into the growing phage filament, which is lipid-free, during assembly. The assembly machinery must catalyze the conversion of protein-phospholipid interactions to protein-protein interactions during the transition from membrane to virion. A strict requirement for coordinated DNA helix translocation across the inner membrane with the association of the major coat protein subunits and dissociation of ssDNA-binding protein pV adds to the complexity of filamentous phage assembly. During this process, DNA serves as an axis around which the coat protein’s helical array is assembled.
Once the DNA has been completely covered by pVIII, the introduction of two other minor polypeptides, pIII and pVI, forms the virion’s terminating cap and releases the phage from the cell. In the absence of pIII or pVI, the infected cell appears to have hundreds of pili-like structures emanating from its surface.
Ff phages have provided the majority of the information about the trans-envelope phage assembly/secretion complex. The assembly is energized at the inner cell membrane; the pI subunit of the pI/pXI complex contains a crucial adenosine triphosphate (ATP)-binding Walkermotif; additionally, it was demonstrated in a semipermeable assembly system that the assembly needs both the ATP and proton-motive force. The trans envelope assembly machinery is completed by pIV, a large outer membrane channel protein that interacts with the pI/pXI complex. pIV is a member of the secretin family, which includes the outer membrane constituents of type II and type III secretion systems, and also the type-IV pilus assembly system. Secretins are large gated channels with periplasmic domains that are extensive. Although phage assembly is a more complex process than producing proteins, the filamentous phage assembly complex, which contains only three proteins, is remarkably simple in comparison to the type II and III secretion systems, which have 15 or more different proteins components. Many lysogenic filamentous phages lack an outer membrane channel and instead rely on secretins from the host type II secretion system or type IV pilus assembly system, which ‘moonlight’ as phage secretins and presumably work with the phage-encoded pI/pXI inner membrane complex.
Applications of filamentous phages
Even though these phages are non-lytic, they can still be useful. Filamentous bacteriophages play an important role in studying biological mechanisms that extend far beyond their own life cycle due to their relative simplicity and ease of genetic manipulation. Filamentous phages are used in the biotechnology industry for a variety of purposes, including:



Comments